Figure 4. Site-specific Gene Editing of Autologous Hematopoietic Stem Cells for Gene Therapy of PID.
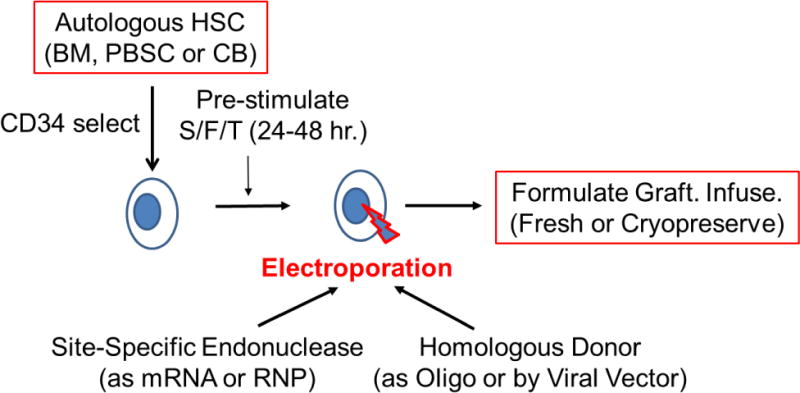
Autologous hematopoietic stem cells (HSC) can be obtained from bone marrow (BM), mobilized peripheral blood stem cells (PBSC) or umbilical cord blood (CB). Usually the HSC are enriched using CD34 immunoselection. The HSC are pre-stimulated to improve gene modification by culture for 24-48 hours with a combination of recombinant hematopoietic growth factors, such as ckit ligand/flt-3 ligand and thrombopoietin (S/F/T). The cells are then treated with electroporation with the site-specific endonuclease (zinc finger nuclease, homing endonuclease, TALEN or CRISPR), to introduce a double stranded break at the target gene, delivered as either in vitro transcribed messenger RNA (mRNA) or pre-formed ribonucleoprotein (RNP) containing the Cas9 protein and a short-guide RNA for CRISPR-mediated processes. The homologous donor containing the corrective sequences may be provided either as an oligonucleotide (Oligo) that is co-electroporated with the endonuclease or via a viral vector (e.g. adeno-associated virus {AAV} or integrase defective lentivirus {IDLV}). Following electroporation, the gene-modified HSC are formulated for intravenous administration and may either be infused into the patient fresh or after cryopreservation and thawing.
