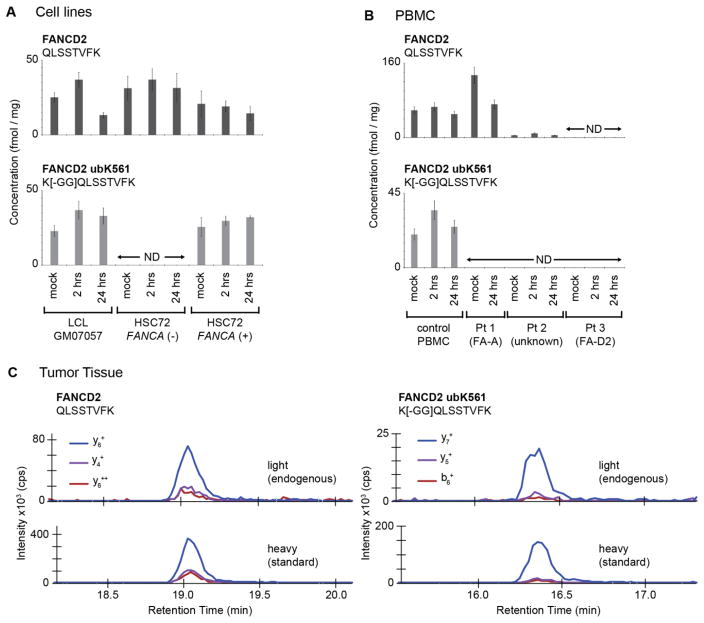
Fig. 2.
Quantification of total and monoubiquitinated FANCD2 in human-derived cell lines and primary human cells and tissue. (A) Concentration of monoubiquitinated FANCD2 peptide and unmodified FANCD2 peptide in human LCLs treated with MMC. Error bars are the standard deviation of biological triplicates or the expected error based on the characterized repeatability of the assay. ‘ND’ is not detected (i.e. below the lower limit of quantification). The FA patient complementation group assignment, where known, is indicated in parentheses. (B) Concentration of monoubiquitinated FANCD2 peptide and unmodified FANCD2 peptide in primary human PBMCs treated with MMC. Error bars are the expected error based on the characterized total CV of the assays. ‘ND’ is not detected (i.e. below the lower limit of quantification). (C) MRM transition ion chromatograms for light (endogenous) and heavy (internal standard) peptides detected in human breast tumor tissue. Specificity is confirmed by detection of multiple transitions from each of the endogenous and heavy-labeled standard peptides, as well as equivalent retention times and relative intensities of transitions for light and heavy peptides.