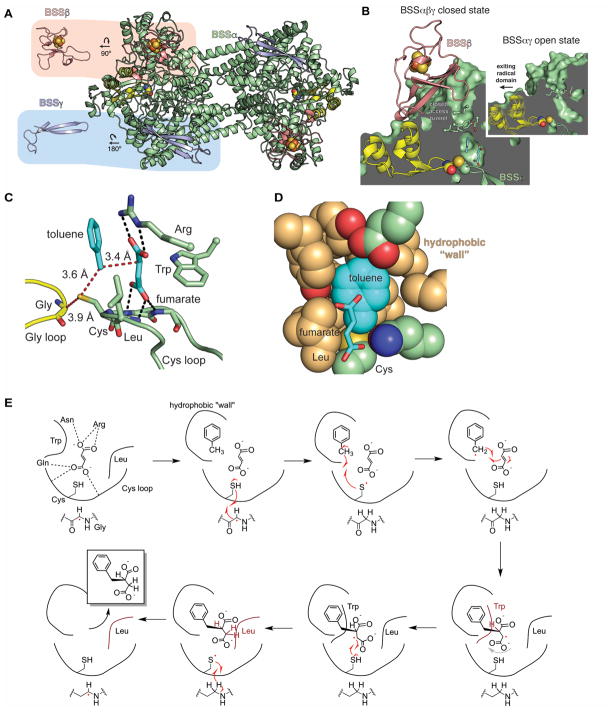
Figure 7. Structure and mechanism of BSS.
A. The BSS heterohexamer is formed by a central BSSα dimer (green with Gly radical domain in yellow) and associated BSSβ (salmon) and BSSγ (light blue) monomers. BSSβ contains a [4Fe-4S] cluster (spheres) whereas BSSγ is partially disordered in the crystal structure and is missing its cluster. B. BSSβ (salmon) contacts the Gly radical domain (yellow) and juts into the putative substrate access channel (green). In the structure without BSSβ (inset), the access channel is more open and the Gly radical domain is shifted away from the Cys loop (closed state shown as faded model, open state as solid). C. The active site of BSS positions the substrates toluene and fumarate such that the expected radical transfer distances (red dashes) are minimized. Hydrogen bonds (black dashes) and van der Waals contacts secure fumarate into place above the Cys loop. D. Van der Waals interactions from the “hydrophobic wall” secure toluene positioning. E. Proposed structure-based mechanism for BSS (Funk et al., 2015). A color version of this figure is available online.