Abstract
Background: Coinfection with HIV/HCV is associated with more severe liver disease, including increased frequency of steatosis and significant fibrosis, compared to patients mono-infected with HCV or HIV. We sought to explore the impact of steatosis on cardiovascular disease (CVD), liver-related outcomes, and survival.
Methods: An IRB-approved, single-center retrospective cohort study was undertaken to analyze 10-year clinical outcomes in HIV/HCV-coinfected patients. Liver biopsy was performed at study entry for the evaluation of HCV disease; a study pathologist graded samples for fibrosis and steatosis. Clinical outcomes, including cardiac events, liver function with FIB-4, AST to Platelet Ratio Index, and survival were assessed over 10 years.
Results: At cohort entry N = 105, mean age 45 ± 7 years, 70% male, and 56% had steatosis present on biopsy. During the 10-year follow-up, no association was found between incident CVD, changes in noninvasive liver fibrosis measures, or survival in the steatosis group compared to nonsteatosis group. However, nonsignificant trends were noted. Overall, mortality for this coinfected population was 25% over 10 years, with liver disease as the most common cause of death.
Conclusions: Given the prevalence of steatosis in approximately half of coinfected patients, larger studies are warranted to determine if steatosis is associated with cardiac disease, diabetes, or liver disease progression in this population. Furthermore, 10-year mortality for this population was very high, underscoring the importance of HCV treatment and need for a better understanding of other variables responsible for decreased survival in this population.
Introduction
Coinfection with HIV/HCV is associated with more severe liver disease, including increased frequency of steatosis, as well as more advanced fibrosis compared to patients mono-infected with HCV or HIV.1,2 In cross-sectional studies of HCV-infected patients, steatosis has consistently been associated with more advanced fibrosis.3,4 In other populations, an association between steatosis and cardiovascular disease (CVD) has been observed, independent of traditional risk factors,5–7 although the relationship between steatosis and cardiovascular outcomes requires further elucidation. We sought to explore the impact of steatosis upon clinical outcomes, including CVD over time, as well as fibrosis progression, liver-related outcomes, and survival.
Materials and Methods
An IRB-approved, single-center retrospective cohort study was undertaken to analyze 10-year clinical outcomes in patients coinfected with HIV and HCV previously studied for histological findings and clinical characteristics associated with hepatic steatosis.1 Included patients underwent liver biopsy between January 1998 and June 2003 for the evaluation of HCV disease. Biopsy samples were assessed by a blinded study pathologist for fibrosis and steatosis with a hepatitis scoring system previously described by Scheuer.8 Clinical outcomes, including cardiac events, new diagnosis of diabetes, liver-related outcomes, and survival, were collected by review of electronic medical records for 10 years following the date of the liver biopsy. Patients lost to follow-up were censored from the time of their last known clinical assessment. CVD was defined as myocardial infarction, coronary artery disease, percutaneous coronary intervention, peripheral vascular disease, or stroke. Diabetes was defined as HbA1c >6.5% or persistent hyperglycemia requiring hypoglycemic or insulin therapy. Liver fibrosis progression was assessed using Fibrosis-4 (FIB4) and AST to Platelet Ratio Index (APRI) scoring systems, with thresholds of 3.25 and 1.5, respectively, defined as cirrhosis for this analysis as previously described.9,10 FIB4 and APRI scores were calculated at the time of enrollment and at the 10-year time point, or time closest to 10 years after enrollment if the patient died or was lost to follow-up. Liver decompensation was defined as clinical signs of ascites, bleeding varices, encephalopathy, or jaundice. Causes of death were obtained from chart review and public social security death indices. Statistical analysis was performed to assess for differences in cardiovascular outcomes, incidence of diabetes, fibrosis scoring, and survival between steatosis and nonsteatosis groups. Based on historical data, we estimated a 0.4 effect size for steatosis and the risk of 10-year cardiovascular and diabetes outcomes so that with a population of n = 80, estimated power = 0.887 with an alpha of 0.05. Data were analyzed using STATA software (version 13.0; StataCorp).
Results
There were 105 patients who met criteria for this study. At cohort entry, mean age was 45 years, 70% of subjects were male, 88% were on ARVs, 61% had undetectable HIV RNA levels, median CD4+ count was 410 cells/mm3, and 11% of patients had CD4+ count <200 cells/mm.3. The mean BMI was 26.3, 10.5% of subjects had diabetes, 21% had hypertension, and 8% had hyperlipidemia (Table 1). Baseline FIB4 and APRI scores were median 2.01 (interquartile range [IQR] 1.12–3.30) and 0.67 (IQR 0.39–1.20) with 27% and 16% meeting score threshold for cirrhosis, respectively. Based on liver biopsy sample analysis, 59 patients had steatosis and 46 did not have steatosis. Fibrosis staging was classified as “mild” (stage 0–1 fibrosis) for 41 patients, “moderate” (stage 2 fibrosis) for 40 patients, or “severe” (stage 3–4 fibrosis) for 24 patients.
Table 1.
Baseline Characteristics by Steatosis Status
| Baseline characteristics | No steatosis (N = 46) | Steatosis (N = 59) | p |
|---|---|---|---|
| Sex, male | 28 (60%) | 44 (74%) | .145 |
| Mean age, years (range) | 44 (30–59) | 46 (34–63) | .127 |
| Overweight/obese | 9 (20%) | 17 (29%) | .363 |
| HCV Genotype 1 | 41 (89%) | 47 (80%) | .286 |
| Any HCV treatment | 14 (30%) | 25 (42%) | .229 |
| AIDS diagnosis | 29 (63%) | 31 (53%) | .324 |
| Hyperlipidemia | 4 (9%) | 4 (7%) | .727 |
| Hypertension | 13 (28%) | 9 (15%) | .147 |
| Any EtOH use | 34 (74%) | 40 (68%) | .526 |
| Diabetes | 4 (9%) | 7 (12%) | .752 |
| Fibrosis stage on biopsy | |||
| None-mild (Stage 0–1) | 23 (50%) | 18 (31%)* | .047* |
| Moderate (Stage 2) | 16 (35%) | 24 (41%) | .552 |
| Severe (Stage 3–4) | 7 (15%) | 17 (29%) | .109 |
| Baseline FIB4, median (IQR) | 1.89 (IQR 1.03–2.88) | 2.04 (IQR 1.12–3.31) | .246 |
| Baseline APRI, median (IQR) | 0.62 (IQR 0.35–0.97) | 0.71 (IQR 0.43–1.24) | .303 |
Statistical significance for p < .05.
APRI, AST to Platelet Ratio Index; FIB4, Fibrosis-4; IQR, interquartile range.
At 10-year follow-up, 52 (50%) subjects were alive, 26 (25%) were deceased, and 27 (25%) were lost to follow-up. Average length of follow-up was 7.5 years (range 1.7–10 years). Of 13 patients with CVD, there were 11 case incidents during the study period: five had clinically significant peripheral vascular disease, four had coronary artery disease, two had myocardial infarction, and two had stroke. Prior to study enrollment one patient suffered MI 6 years prior to biopsy, with two subsequent MI events during the 10-year study period, and one patient had preexisting PVD with ulceration. By 10-year follow-up, 18 patients (17%) had diabetes, of which 8 (7.6%) were a new diagnosis over the study period.
Steatosis and clinical outcomes
Analysis of cardiovascular and metabolic outcomes by steatosis status showed numerically higher, but nonsignificant rates of diabetes (22%), myocardial infarction (5%), coronary artery disease (5%), and peripheral vascular disease (5%) in the steatosis group compared to those without steatosis (11%, 2%, 4%, and 4%, respectively) over the 10-year period (p = .67, p = .14, and p = .31, respectively). Steatosis was also not significantly associated with a new diabetes diagnoses (p = .64).
Analysis of liver-related outcomes showed median FIB4, and APRI scores were calculated at the time of enrollment and at the time point closest to 10 years after liver biopsy. Median FIB4 and APRI scores were not significantly higher in the steatosis group compared to the nonsteatosis group at baseline (FIB4 2.04 vs. 1.89, p = .31, and APRI 0.71 vs. 0.62, p = .45) and the 10-year time point (FIB4 2.47 vs. 2.16, p = .12, and APRI 0.73 vs. 0.59, p = .14). However, a relationship exists when the analysis was limited to those with some fibrosis at baseline (p = .04, p = .06). A total of 18 patients developed decompensated liver disease and two patients with decompensated liver disease developed hepatocellular carcinoma (HCC) during the follow-up period. Of patients with decompensated liver disease, 61% had steatosis at the time of biopsy.
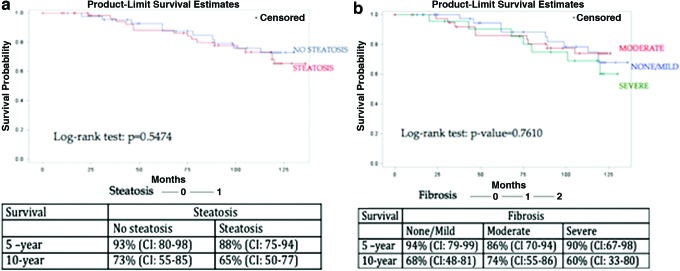
Survival analysis showed no significant difference (HR = 1.27, p = .56) in survival between the steatosis group at the 5- and 10-year time points with 5-year survival at 88% and 10-year survival at 65% in the steatosis group versus 93% and 73% at respective time points in the nonsteatosis group. Figure 1a shows survival rates by baseline steatosis.
FIG. 1.
(a) Survival, defined as no mortality, by steatosis status from baseline liver biopsy pathology. (b) Survival, defined as no mortality, by fibrosis status from baseline liver biopsy pathology. Color images available online at www.liebertpub.com/aid
Fibrosis and survival
Analysis by baseline fibrosis stage seen on biopsy, showed the group with no fibrosis had 94% 5-year survival and 68% 10-year survival; the group with mild fibrosis had 86% 5-year survival and 74% 10-year survival; and the group with moderate/severe fibrosis had 90% 5-year survival and 60% 10-year survival (Fig. 1b). Change in FIB4 and APRI was also assessed for impact upon survival. Change in FIB4 has an unadjusted HR = 1.081 (p = .0001), and change in APRI has an unadjusted HR = 1.151 (p < .0001). These relationships remained highly significant after adjustment for potential interacting factors, including alcohol use, age, diabetes, obesity, or hyperlipidemia.
Causes of death
Of the 25% of subjects who died during this study, mean age at death was 52.9 years; 10 patients died due to decompensated liver disease, including one patient with HCC as cause of death. Five patients died due to sepsis, three due to other malignancy, and 10 from other/unknown causes (eight unknown, one ESRD/CHF, and two drug overdose).
HCV treatment
Forty-seven (45%) of the 105 patients underwent some form of interferon-based HCV therapy. Of the patients treated, 11 (10%) were assessed to have clinical response. Patients with a response to treatment were found to have lower mean APRI scores than the nonresponders/untreated (0.49 vs. 1.73, p = .013), but did not differ by steatosis status. Ten-year survival was 58% in patients who received treatment and 43% in patients who did not receive treatment, which was not statistically significant.
Discussion
This retrospective cohort study in HIV/HCV-coinfected patients did not detect a significant association between histologically proven steatosis and survival, cardiovascular events, or diabetic events. However, the data trended toward an increased rate of cardiovascular events, diabetes, and decreased survival in patients with steatosis. It is possible that no association was seen because the underlying etiology of steatosis differs in HIV/HCV-coinfected patient compared to NAFLD, where it has been shown to be an independent risk factor for development of CVD. However, in argument against this, traditional risk factors for fatty liver were identified in the original study of this cohort (i.e., elevated BMI and low HDL). Heterogeneity of the cohort's liver disease at baseline is a limit to this study. Given the natural history of NAFLD that steatosis regresses with development of cirrhosis, patients with cirrhosis at baseline may have had NAFLD but not display steatosis on biopsy. However, perhaps most importantly, low numbers of observed CV events, as well as high mortality and lost to follow-up, may have resulted in inadequate power to discern a difference due to the relatively small total sample size.
The 10-year mortality of 25% was strikingly high given a median baseline mean age of 45 for this cohort at the time of study enrollment, and change in fibrosis markers (FIB4 and APRI) was associated with poor survival. Accumulating evidence demonstrates significant reductions in mortality and morbidity in patients infected with HIV in the United States since the introduction of combination antiretroviral therapy in 1996; however, HIV/HCV coinfection consistently predicts increased mortality.11–14 The treatment for HCV has evolved greatly in the 15 years since this cohort was assembled, and with the availability of direct acting antiviral therapy, HCV is now a curable condition. Inadequate numbers were successfully treated in this study to assess for an association between sustained virologic response and survival; however, a recent large cohort study showed a hazard ratio of 0.21 for HCV-related outcomes in those treated successfully, as well as reduced rate of HIV progression.15
In summary, steatosis rates were high in this cohort of HIV/HCV-coinfected outcomes, but did not significantly predict 10-year cardiovascular outcomes or survival, but an effect may have been obscured by high rates of loss to follow-up, high mortality, and a relatively small sample size. Nonetheless, given the prevalence of steatosis in approximately half of HIV/HCV-coinfected patients, the trends in cardiovascular, fibrosis progression, and survival differences observed over 10 years warrant further study. The low survival rates and association between liver disease markers and survival underscore the importance of HCV treatment in HIV/HCV-coinfected individuals, as most deaths were due to liver disease in this cohort.
Statistical support
CTSC Statistical Support: grant UL1 TR000457.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Marks KM, Petrovic LM, Talal AH, Murray MP, Gulick RM, Glesby MJ: Histological findings and clinical characteristics associated with hepatic steatosis in patients coinfected with HIV and hepatitis C virus. J Infect Dis 2005;192:1943–1949 [DOI] [PubMed] [Google Scholar]
- 2.Gaslightwala I, Bini EJ: Impact of human immunodeficiency virus infection on the prevalence and severity of steatosis in patients with chronic hepatitis C virus infection. J Hepatol 2006;44:1026–1032 [DOI] [PubMed] [Google Scholar]
- 3.Castera L, Hezode C, Roudot-Thoraval F, et al. : Worsening of steatosis is an independent factor of fibrosis progression in untreated patients with chronic hepatitis C and paired liver biopsies. Gut 2003;52:288–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leandro G, Mangia A, Hui J, et al. : Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: A meta-analysis of individual patient data. Gastroenterology 2006;130:1636–1642 [DOI] [PubMed] [Google Scholar]
- 5.Lin Y-C, Lo H-M, Chen J-D. Sonographic fatty liver, overweight and ischemic heart disease. World J Gastroenterol 2005;11:4838–4842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamaguchi M, Kojima T, Takeda N, et al. : Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol 2007;13:1579–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Targher G, Bertolini L, Padovani R, et al. : Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007;30:1212–1218 [DOI] [PubMed] [Google Scholar]
- 8.Scheuer PJ. Classification of chronic viral hepatitis: A need for reassessment. Journal of hepatology 1991;13:372–374 [DOI] [PubMed] [Google Scholar]
- 9.Sterling RK, Lissen E, Clumeck N, et al. : Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–1325 [DOI] [PubMed] [Google Scholar]
- 10.Wai C-T, Greenson JK, Fontana RJ, et al. : A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–526 [DOI] [PubMed] [Google Scholar]
- 11.Alejos B, Hernando V, López-Aldeguer J, et al. : Overall and cause-specific mortality in HIV-positive subjects compared to the general population. J Int AIDS Soc 2014;17:19711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greub G, Ledergerber B, Battegay M, et al. : Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: The Swiss HIV Cohort Study. Lancet 2000;356:1800–1805 [DOI] [PubMed] [Google Scholar]
- 13.Weber R, Sabin C, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S, et al. : Liver-related deaths in persons infected with the human immunodeficiency virus: The D:A:D study. Arch Intern Med 2006;166:1632–1641 [DOI] [PubMed] [Google Scholar]
- 14.Berenguer J, Zamora FX, Carrero A, et al. : Effects of sustained viral response in patients with HIV and chronic hepatitis C and nonadvanced liver fibrosis. J Acquir Immune Defic Syndr 2014;66:280–287 [DOI] [PubMed] [Google Scholar]
- 15.Smith C, Sabin C, Lundgren JD, Thiebaut R, Weber R, Law M, Monforte Ad, Kirk O, Friis-Moller N, Phillips A, et al. : Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 2010;24:1537–1548 [DOI] [PubMed] [Google Scholar]