Figure 3. Gal8 is in dynamic complexes with mTOR and its regulators and adaptors.
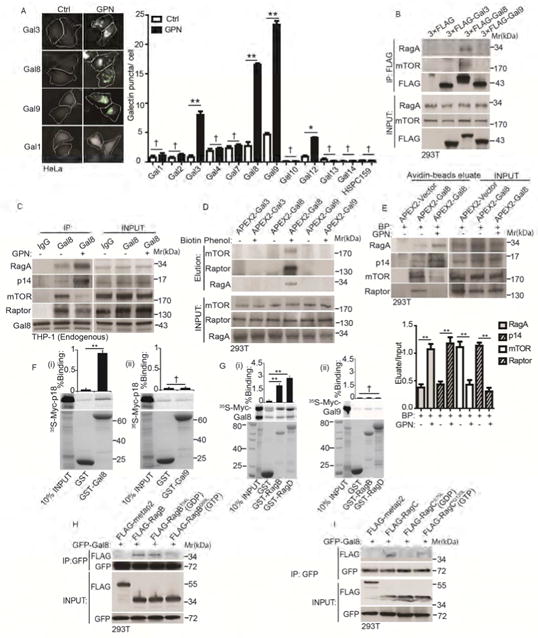
(A) Galectin puncta formation in response to GPN. Cells expressing YFP-galectin fusions were treated with 100 μM GPN or without (Ctrl) in full medium for 1 h and galectin puncta quantified by HC. Left, images of galectins 1, 3, 8, and 9. White masks, algorithm-defined cell boundaries (primary objects); green masks, computer-identified galectin puncta (target objects). (B) Co-immunoprecipitation (Co-IP) analysis of galectins and mTOR or RagA. Cells expressing FLAG-tagged galectins were subjected to anti-FLAG immunoprecipitation followed by immunoblotting for endogenous mTOR or RagA. (C) Co-IP analysis of endogenous proteins in macrophage-like cells treated with 100 μM GPN in full medium1 h. IP: anti-Gal8; immunoblotting: endogenous RagA, p14, mTOR and Raptor. (D) APEX2 proximity biotinylation analysis. Cells were transfected with APEX2 fusions with Gal3, 8 and 9, incubated or not with biotin-phenol, pulsed with H2O2, and biotinylated proteins affinity-isolated on streptavidin-beads analyzed by immunoblotting. (E) Proximity biotinylation as in D in response to GPN. BP, biotin-phenol. (F)(i-ii) GST pulldown assay of in vitro translated and radiolabeled Myc-tagged p18 with GST, or GST-tagged Gal8 and Gal9. Data (% binding). (G)(i-ii) GST pulldown assay of in vitro translated Myc-tagged Gal8 or Gal9 with GST or GST-tagged RagB/D. Data as in F. (H) Cells transfected with GFP-Gal8 and FLAG-tagged metap2 (negative control) or RagB variants (RagBWT, RagBT54L or RagBQ99L) were subjected to anti-GFP IP, followed by immunoblotting for FLAG-tagged proteins or GFP. (I) Cells transfected with GFP-Gal8 and FLAG-tagged metap2 or RagC variants (RagCWT, RagCS75L or RagCQ120L) processed as in H; immunoblotting: FLAG or GFP. Data, means □ SEM; blots: n ≥ 3, HC: n ≥ 3 (each experiment: 500 valid primary objects/cells per well, ≥ 5 wells/sample). † p ≥ 0.05 (not significant), *p < 0.05, **p < 0.01, ANOVA. See also Figure S3.
