Table 1. Optimization of reaction parameters a .
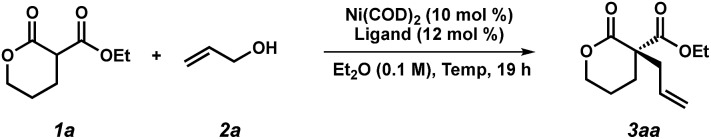
| ||||
| Entry | Ligand | Temp (°C) | % Yield b | % ee c |
| 1 | L1 | 0 | 53 | 75 |
| 2 | L2 | 0 | 76 | 78 |
| 3 | L3 | 0 | 93 | 79 |
| 4 | L4 | 0 | 82 | 82 |
| 5 d | L4 | 0 | 62 | 81 |
| 6 | L4 | 23 | 86 | 74 |
| 7 | L4 | –10 | 69 | 84 |
| 8 e | L4 | –10 | 80 | 85 |
| 9 | — | 0 | 0 | — |
| ||||
aConditions: 1a (0.1 mmol), 2a (0.1 mmol), Ni(COD)2 (10 mol%), ligand (12 mol%) in Et2O (1.0 mL).
bYields determined by 1H NMR of crude reaction mixture using 1,3,5-trimethoxybenzene as a standard.
cDetermined by chiral SFC analysis of the isolated product.
dNi(COD)2 (5 mol%) and L4 (6 mol%) were used.
eReaction time = 48 h.
