Abstract
Background:
Gastrointestinal (GI) events are common adverse events (AEs) associated with delayed-release dimethyl fumarate (DMF), an approved treatment for relapsing–remitting multiple sclerosis (RRMS). The objective of the TOLERATE study was to evaluate GI tolerability and GI mitigation via symptomatic therapies in patients initiating DMF in a real-world clinical setting in Germany.
Methods:
TOLERATE was a multicentre, open-label, single-arm study performed at 25 German sites. Endpoints were frequency, severity, duration (all primary) and mitigation of GI-related events (secondary). Patients were instructed to take DMF according to the prescribing information for up to 12 weeks and to document GI events and intake of GI-symptomatic therapy on numerical rating scales, using eDiaries.
Results:
A total of 211 patients were included in the safety population (71% female; mean age 40 ± 11 years). Of these, 185 patients (87.7%) reported GI-related events, out of which nearly half received GI-symptomatic therapy (84/185; 45.4%). The most frequently reported GI events were upper abdominal pain, flatulence and nausea. GI-related events peaked during the first 3 weeks of therapy and rapidly decreased thereafter. The severity of GI events over 12 weeks according to the Modified Overall Gastrointestinal Symptom Scale were mild to moderate in the majority of patients reporting GI-related events and taking symptomatic GI medication (53.6%). Only 10% of all patients discontinued study treatment due to AEs in general, while 6.6% discontinued due to GI-related events. The severity of GI-related events decreased over time in patients who received symptomatic treatment with one or more medications (e.g. acid secretion blockers, antidiarrhoeals or antiemetics).
Conclusion:
Gastrointestinal events associated with delayed-release DMF were mainly mild to moderate in severity. Prevalence of GI events peaked during the first 3 weeks of therapy and rapidly faded thereafter. Although 44.9% of patients experiencing GI events used common GI symptomatic therapies, only 6.6% of patients discontinued DMF because of GI events, suggesting that GI events could be managed well with common symptomatic therapy.
Keywords: dimethyl fumarate, gastrointestinal events, multiple sclerosis
Introduction
The primary treatment goal in multiple sclerosis (MS) is to reduce the frequency and severity of relapses and to delay the progression of the disease. The range of disease-modifying treatment options has expanded in recent years, with new entrants including injectable therapies, infusions, as well as oral formulations.1–3,10,12,16
Delayed-release dimethyl fumarate (DMF) (Tecfidera®) was approved in the US in 2013 and in the European Union in 2014 for the treatment of patients with relapsing–remitting MS (RRMS). The mechanism by which DMF exerts its therapeutic effect in MS is yet unknown but may involve activation of the nuclear factor (erythroid-derived 2)-related factor 2 pathway (Nrf2 pathway), which is involved in the cellular response to oxidative stress, a major pathologic factor in MS.4–6
The clinical efficacy of DMF, as evidenced by significant reductions on measures of relapse and disability progression in patients with RRMS receiving 240 mg twice or thrice daily, was demonstrated in three placebo-controlled clinical trials.7–9 While the overall safety profile of oral DMF was favourable in these clinical studies, between 27% and 38% of DMF-treated patients experienced gastrointestinal (GI)-related adverse events (AEs) within the first 3 months of treatment.8,9 The incidence of these AEs was highest during the first month of treatment and decreased in the second and subsequent months of treatment, with the majority of patients reporting GI-related AEs of mild-to-moderate severity. Nevertheless, there was a higher incidence of study discontinuations due to GI events in DMF-treated patients (⩽5%) compared with placebo-treated patients (⩽2%). Between 11% and 16% of these patients utilized GI-related symptomatic therapies.7–9 However, it is unclear whether symptomatic therapies were taken in response to GI-related AEs that occurred at the time of dosing and whether the therapies were effective.
TOLERATE (a multicentre open-label, single-arm study to evaluate gastrointestinal TOLerability in patients with rElapsing–Remitting multiple sclerosis receiving dimethyl fumarATE) examined the frequency, severity and duration of GI-related events, as well as the use and effect of symptomatic GI medication, in RRMS patients treated with DMF in a real-world clinical setting in Germany.
Methods
Study design
This multicentre, open-label, single-arm phase IV study was conducted in 25 neurological sites involved in diagnosis and care of MS patients in Germany from June 2014 to March 2016. The observation period per patient was 14 weeks, including four visits to the study site during treatment and a safety follow-up telephone interview.
The objectives of TOLERATE were to examine the incidence, severity and duration of GI-related events in DMF patients and to describe the impact of symptomatic therapy in a clinical practice setting. TOLERATE also examined the effects of dosing with food and dose modifications on GI-related events. Additionally, serum biomarkers associated with methyl group metabolism were examined in a proof-of-concept approach for the prediction of possible GI events.
The study was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice guidelines, the approval of all relevant institutional review boards and ethics committees and local regulatory requirements. Ethics approval was obtained from the ethics committee of the Ruhr University, Bochum (No. 4960-14FF). Before enrolment, all participants provided written informed consent.
Participants
Patients were enrolled across 25 study sites in Germany. Patients meeting the following selection criteria were eligible to participate in the study: (a) ability to understand the purpose and risks of the study and provide signed and dated informed consent in accordance with national and local patient privacy regulations; (b) a confirmed diagnosis of RRMS according to the revised McDonald Criteria10 and satisfying the therapeutic indication as described in the official local registration for DMF; (c) age ⩾18 years at the time of informed consent; and (d) naïve to DMF and fumaric acid esters (e.g. all methyl and ethyl fumarate derivatives). Exclusion criteria were: (a) inability to comply with study requirements or, at the discretion of the investigator, deemed unsuitable for study participation; (b) female patients who were currently pregnant or breastfeeding, or who were considering becoming pregnant in the near future; (c) a history of significant GI disease (e.g. irritable bowel disease, peptic ulcer disease, history of major GI surgeries) or chronic use of GI-related symptomatic therapy as determined by the investigator, or ⩾7 consecutive days of GI-related symptomatic therapy (e.g. loperamide hydrochloride, omeprazole, esomeprazole, lansoprazole, pantoprazole, paracetamol, acetylsalicylic acid, ranitidine, loratadine, butylscopolamine bromide, metoclopramide, domperidone, or dimethicone within the month prior to enrolment in the study); (d) presence of one or more major comorbidities that, in the opinion of the investigator, may have affected the outcome of the study; (e) known active malignancies (patients with cutaneous basal cell carcinoma that has been completely excised prior to study entry remain eligible); (f) history of anaphylaxis or severe allergic reactions or known drug hypersensitivity; (g) current enrolment in any clinical trial except the DMF Pregnancy Exposure Registry or other studies that, according to the study medical director, would not conflict with the study (e.g. health economics studies or local registries); (h) current use of B vitamin supplements; and (i) in the opinion of the investigator, blood test values suggestive of a low lymphocyte count or renal or hepatic impairment, as described in the locally applicable prescribing information precautions for use.
Procedures
After the baseline visit, follow-up visits were scheduled at weeks 4, 8 and 12; telephone contact was permissible at week 8, if necessary. A safety follow-up telephone interview was conducted 2 weeks (±5 days) after the final dose of DMF. Blood samples for biomarker analysis were collected at the baseline visit and at week 12. Each patient’s capacity for methyl group metabolism was examined through assays of serum pyridoxal phosphate (vitamin B6), folic acid (vitamin B9), cobalamin (vitamin B12) and homocysteine.
All patients were instructed to take DMF over a 12-week period according to the prescribing information, which indicates a starting dose of 120 mg twice a day (BID) for 7 days followed by an increase to the recommended dose of 240 mg BID. As outlined in the prescribing information, patients were advised to take DMF with food, that is, with a meal or within 1 h after a meal, in order to mitigate GI-related events. GI events were self reported by participants using an eDiary containing two questionnaires designed to obtain detailed information on the incidence, severity, duration and onset of GI symptoms. Global GI events (defined as one or more of the following symptoms like nausea, diarrhoea, upper abdominal pain, lower abdominal pain, vomiting, indigestion, constipation, bloating and flatulence) and their effect on the patient during the 24 h before each morning dose were assessed on the Modified Overall Gastrointestinal Symptom Scale (MOGISS). Acute GI-related symptoms (nausea, diarrhoea, upper abdominal pain, lower abdominal pain, vomiting, indigestion, constipation, bloating and flatulence) that the patient experienced during the 10 h after each morning and evening dose were assessed on the Modified Acute Gastrointestinal Symptom Scale (MAGISS). In both questionnaires, items were rated on a 10-point numerical rating scale (0 = no events, 1 to 3 = mild events, 4 to 6 = moderate events, 7 to 9 = severe events and 10 = extreme events).11 In addition, patients were instructed to document intake of all symptomatic therapies. All adverse events (serious and nonserious) were documented (excluding those of MS relapse that did not lead to permanent treatment discontinuation or study withdrawal).
Outcomes
The primary endpoint was the frequency, severity and duration of GI-related events in DMF-treated patients with RRMS who utilized symptomatic therapy during the 12-week treatment period. Secondary endpoints were the cumulative proportion of patients who required GI symptomatic therapy, the patient-reported frequency and duration of symptomatic therapies, the cumulative proportion of patients who required DMF dose reduction in response to GI-related events and the percentage of patients who discontinued DMF due to GI-related events.
Statistical analysis
Due to the exploratory nature of the study, no formal sample size calculation was performed. The study sample size of 230 was based on the sample size included in a similarly conducted study in the US.11 All data analyses were carried out according to a pre-established analysis plan. All data were summarised using descriptive statistics. Analyses of frequency, severity and duration of GI-related events in DMF-treated patients with RRMS were based on scales grading the overall and specific features of the events11 (MOGISS, MAGISS). The worst severity scores reported were summarised by group and analysis period. Total and average duration for use of symptomatic therapies were calculated. The cumulative proportion of DMF-treated patients with RRMS who required symptomatic therapy up to week 12 and the cumulative proportion of patients who underwent a DMF dose modification in response to GI-related events during the 12-week treatment period were estimated using the Kaplan–Meier method. Here, we present the results from the safety population, defined as patients who received at least one dose of DMF.
Results
Patient disposition and baseline characteristics
A total of 214 patients were screened and enrolled into the study. One patient withdrew consent and two patients did not meet the inclusion/exclusion criteria and were excluded from the safety population. In total, 211 patients were included in the safety population, which comprised patients aged 18–62 years, with a mean (±SD) age of 40.1 ± 11 years. The majority of patients (70.6%) were female. Baseline demographics are summarised in Table 1.
Table 1.
Baseline demographics and multiple sclerosis treatment history.
| Baseline demographics | n = 211 |
|---|---|
| Age, mean (SD), years | 40.1 (11) |
| Females, n (%) | 149 (70.6%) |
| MS duration, mean (SD), years | 6.4 (6.9) |
| Days from the most recent relapse, mean (SD) | 426.7 (698.6) |
| MS treatment duration, mean (SD), months | 30 (44.8) |
| Total number of relapses within past 6 months, mean (SD) | 0.7 (0.7) |
| Patients with prior MS treatment, n (%) | 159 (75.4%) |
| GI history prior to baseline | 6 (2.8%) |
| Previous MS treatments used, n (%)* | |
| Chronic MS-related steroid | 55 (26.1%) |
| Interferon beta-1a | 50 (23.7%) |
| Glatiramer acetate | 28 (13.3%) |
| Interferon beta-1b | 14 (6.6%) |
| Fingolimod | 10 (4.7%) |
| Natalizumab | 7 (3.3%) |
| Azathioprine | 1 (0.5%) |
| Mitoxantrone® | 1 (0.5%) |
Multiple answers possible.
GI, gastrointestinal; MS, multiple sclerosis; SD, standard deviation.
Incidence of gastrointestinal-related events
A total of 185 patients (185/211, 87.2%) reported acute upper GI-related events on the MOGISS, out of which 84 (84/185, 45.4%) took symptomatic therapy and a total of 191 patients (191/211, 90.5%) reported lower GI-related events, out of which 82 (82/191, 42.9%) took symptomatic therapy. The proportion of patients with and without GI events did not differ substantially across baseline and clinical characteristics.
The most commonly reported GI-related symptoms across the entire observation period of 12 weeks according to the MAGISS were upper abdominal pain, flatulence, nausea, bloating, diarrhoea and lower abdominal pain (Table 2).
Table 2.
Incidence of gastrointestinal-related events* (safety population; n = 211).
| GI-related events in general n/n (%) | GI-related events treated with symptomatic therapy n/n (%) | |
|---|---|---|
| Acute upper GI-related events | 185/211 (87.7%) | 84/211 (39.8%) |
| Upper abdominal pain | 150/211 (71.1%) | 72/211 (34.1%) |
| Nausea | 137/211 (64.9%) | 64/211 (30.3%) |
| Indigestion | 79/211 (37.4%) | 39/211 (18.5%) |
| Vomiting | 2/211(13.7%) | 23/211 (10.9%) |
| Acute lower GI-related events | 191/211 (90.5%) | 82/211 (38.8%) |
| Flatulence | 140/211 (66.4%) | 66/211 (31.3%) |
| Diarrhoea | 135/211 (64%) | 60/211 (28.4%) |
| Bloating | 126/211 (59.7%) | 61/211(28.9%) |
| Lower abdominal pain | 107/211 (50.7%) | 53/211 (25.1%) |
| Constipation | 69/211 (32.7%) | 36/211 (17.1%) |
Some patients reported more than one GI event.
GI, gastrointestinal.
Overall prevalence of GI-related events over the 12-week observation period in patients taking symptomatic GI therapy according to the MOGISS were highest within the first 3 weeks of treatment (24%) and decreased continuously thereafter (Figure 1).
Figure 1.
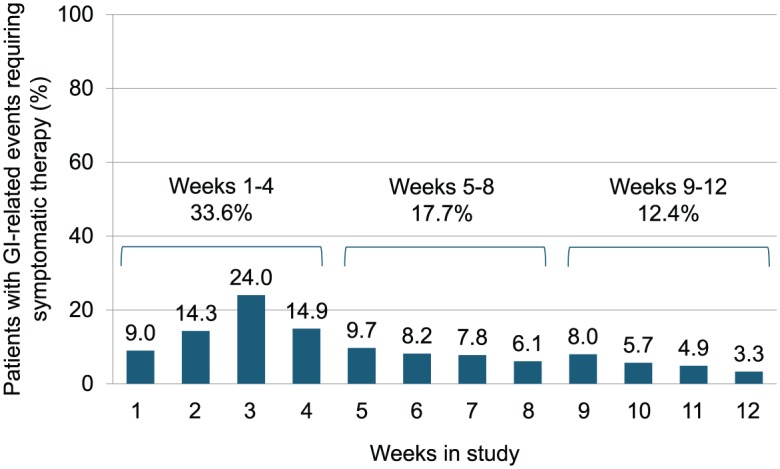
Prevalence of gastrointestinal-related events (Modified Overall Gastrointestinal Symptom Scale) requiring symptomatic therapy (%) over time (safety population; n = 211).
GI, gastrointestinal.
Severity of gastrointestinal-related events
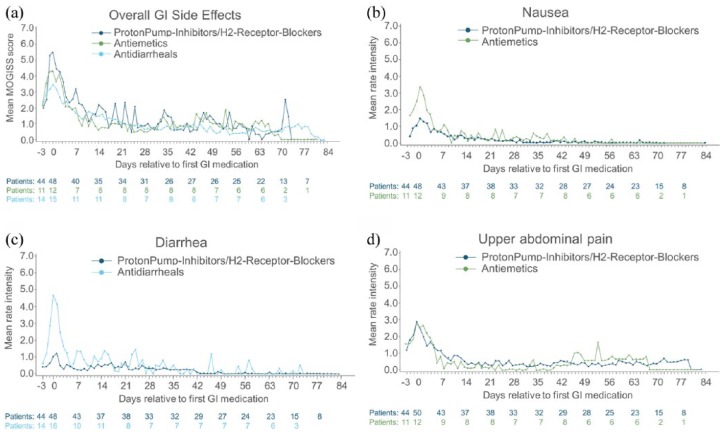
Of the 84 patients with GI events who took symptomatic GI medication, severity grades over 12 weeks according to the MOGISS were reported as follows: 2.4% reported no events, 53.6% mild/moderate, 35.7% severe and 8.3% extreme. Severity grades of GI-related events over time for patients who took symptomatic GI medication are shown in Figure 2(a–d). In general, the severity of GI-related events decreased over time in patients who received treatment with one or more medications from the following categories: acid secretion blockers (proton-pump inhibitors and H2 receptor blockers), antidiarrhoeals (loperamide) and antiemetics (dimenhydrinate, metoclopramide). The mean severity of symptoms according to the MAGISS was highest at the time of first use of GI medication and then decreased until the end of the observation period (84 days after first use of GI medication). For both GI-related events reported on the MOGISS and on the MAGISS, the mean worst severity score peaked at week 3 at 5.2 and 5.7, respectively. For specific GI-related events, the highest mean worst severity scores were seen for upper abdominal pain, nausea and diarrhoea [as shown in Figure 2(b–d)], bloating and flatulence. The mean worst severity scores for GI-related events reported on the MOGISS, observed for the symptomatic therapy types with more than five patients, were as follows: 6.5 ± 2 (n = 50) for events associated with antacid production, 5.6 ± 2.1 (n = 26) for antibloating/anticonstipation agents, 5.9 ± 2.4 (n = 20) for multitarget/herbal agents, 7.5 ± 2.3 (n = 16) for antidiarrhoeal (antiperistaltic) agents, 7.0 ± 1.9 (n = 10) for analgesic [nonsteroidal anti-inflammatory drug (NSAID)] agents, 7.0 ± 1.1 (n = 6) for antiemetic (pro-kinetic) agents and 6.5 ± 3.2 (n = 8) for anti-emetic (central) agents. The mean worst severity score for acute GI-related events reported on the MAGISS for each type of symptomatic therapy type were similar to those seen on the MOGISS. No GI-related serious AEs (SAEs) were reported during the study.
Figure 2.
Severity of gastrointestinal-related events relative to gastrointestinal-symptomatic medications.
(a–d), severity of GI-related events over time in patients who received GI-symptomatic medications according to the MOGISS [Figure 2(a)] and the MAGISS [Figure 2(b–d)].
GI, gastrointestinal; MOGISS, Modified Overall Gastrointestinal Symptom Scale; MAGISS, Modified Acute Gastrointestinal Symptom Scale.
Symptomatic therapy of gastrointestinal events
The most commonly reported symptomatic therapies among patients who took symptomatic medication in the 12-week observation time were antacid production agents (50/84; 59.5%), antibloating/anticonstipation agents (26/84; 36%), multitarget/herbal agents (20/84; 23.8%), spasmolytics (18/84; 21.4%), antidiarrhoeal (antiperistaltic) agents (16/84; 15.9%) and antiemetics (14/84; 16.7%). It should be noted that several patients took more than one GI medication in this time period. Nearly 80% of the patients who took symptomatic therapy initiated a therapy within the first 3 weeks of the study. The percentage of patients who reported symptoms by the MOGISS and took symptomatic therapy peaked at week 3 (24%), and decreased thereafter to 9.7% in week 5 and to 3.3% in week 12 (Figure 1). A similar trend was observed for acute GI events reported on the MAGISS. Across all therapies, the median number of days on symptomatic therapy was 5, ranging from a minimum of 1 day to a maximum of 80 days. The most commonly used GI symptomatic medications are summarized in Table 3.
Table 3.
Most commonly used symptomatic gastrointestinal medication in TOLERATE.
| Symptomatic therapy category | Symptomatic GI medication |
|---|---|
| Antacid production agents | |
| Proton-pump inhibitors | Esomeprazole/omeprazole/pantoprazole |
| H2-secretion blockers | Ranitidine |
| Antidiarrhoeals | Loperamide |
| Antiemetics | Metoclopramide/dimenhidrinate/domperidone |
| Antacids | Ca-carbonate/Na-bicarbonate/Al-oxide |
| Analgesics (NSAIDs) | Ibuprofen/metamizole |
| Antibloating/anticonstipation agents | Simeticon/dimeticon/Movicol/sodium picosulfate |
| Laxatives | Bisacodyl |
| Spasmolytics | Butylscopalamine (hyoscine butylbromide) |
| Multitarget/herbal agents | Iberogast®/Gaviscon®/Wikalin® |
| Others | Carbon tablets/Lactobacillus acidophilus/Saccharomyces boulardii |
Al-oxide, aluminium oxide; Ca-carbonate, calcium carbonate; GI, gastrointestinal; Na-bicarbonate, sodium bicarbonate; NSAIDs, nonsteroidal anti-inflammatory drugs.
Adverse events and study discontinuation
A total of 5/211 patients (2.4%) experienced at least one SAE. Observed SAEs included MS relapse (n = 2), hypersensitivity to DMF (n = 1), alcohol abuse (n = 1) and increased levels of alanine amino transferase, aspartate amino transferase and gamma glutamyl transferase (ALT, AST GGT) (n = 1; all three enzyme-increase events experienced by the same patient). One hypersensitivity reaction to DMF and one event of increased levels of ALT, AST and GGT were considered to be related to DMF and led to permanent discontinuation of DMF and withdrawal from the study.
In total, 31 patients (14.7%) withdrew from the study and discontinued DMF treatment, 21 of these due to adverse events (10%). Of these, 14 (6.6%) discontinued due to GI disorders, comprising a total of 21 events that were all treated symptomatically. A total of 14/21 events were reported to be of moderate severity (6 upper abdominal pain, 3 nausea, 2 each of diarrhoea and vomiting, and 1 case each of flatulence and upper abdominal discomfort), 1 of mild severity (diarrhoea) and 6 as severe: 3 diarrhoea events and 1 event each of nausea, constipation and gastritis (Table 4).
Table 4.
Study discontinuation and adverse events (n = 211).
| n (%) | |
|---|---|
| Patients discontinued study treatment, overall | 31 (14.7%) |
| Patients discontinued study treatment due to AEs | 21 (10%) |
| Patients discontinued study treatment due to GI-related AEs* | 14 (6.6%) |
| Abdominal pain | 6 (2.8%) |
| Diarrhoea | 6 (2.8%) |
| Nausea | 4 (1.9%) |
| Flatulence | 3 (1.4%) |
| Vomiting | 2 (0.9%) |
| Constipation | 1 (0.5%) |
| Gastritis | 1 (0.5%) |
Some patients reported more than one GI adverse event.
AEs, adverse events; GI, gastrointestinal.
Dose reductions of dimethyl fumarate
Among the 211 patients, the proportion of patients who did not titrate fully to the 240 mg twice-daily (BID) dose ranged from 8.5 to 12.8% between days 9 and 15. On day 16, the percentage of patients not taking 240 mg BID was 20.4%, which may have been the result of a dose reduction for some patients.
In all, 71 patients (34.6%) reported a dose reduction. Indeed, 41 of these reported intake of symptomatic GI medication with an average DMF dose reduction for 14 days, whereas 32 patients who did not use symptomatic GI medication had an average on 5 days of reduced DMF dosing. Only one patient received a reduced dose of DMF over the whole observation period.
Association between gastrointestinal-related events and administration of dimethyl fumarate with food
Among the 211 patients in the safety population, 51 (24.2%) reported always taking DMF with food (with a meal or within 1 h after a meal; Figure 3).
Figure 3.
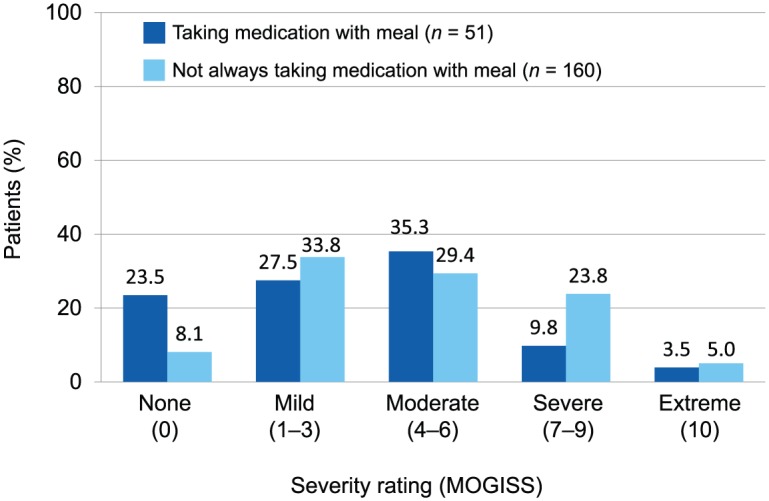
Association between gastrointestinal severity scores (MOGISS) and intake of dimethyl fumarate with a meal (safety population; n = 211).
MOGISS, Modified Overall Gastrointestinal Symptom Scale.
A lower proportion of patients who reported always taking DMF with food reported GI-related events compared with the patients who reported not always taking DMF with food. A total of 39/186 patients (21%) with general GI-related events and 12/25 patients (48%) without general GI-related events per the MOGISS reported always taking DMF with food. Similarly, 42 of 192 patients (21.9%) with acute GI events and 9 of 19 patients (47.4%) without acute GI events per the MAGISS reported always taking DMF with food.
For GI-related events reported on the MOGISS, patients who reported not always taking DMF with a meal (n = 160) had a higher mean worst severity score compared with patients who reported always taking DMF with a meal (n = 51): 4.4 [standard deviation (SD) 2.9] versus 3.3 (SD 2.9) (Figure 3). Similarly, for acute GI-related events reported on the MAGISS, patients who reported not always taking DMF with a meal had a higher mean worst severity score compared to patients who reported always taking DMF with a meal: 4.4 (SD 2.9) versus 3.3 (SD 2.7).
Association between gastrointestinal-related events and vitamin B
Levels of vitamin B6, vitamin B12, homocysteine and folic acid were normal at both baseline and week 12 in 72.4%, 73%, 62% and 39.1% of patients, respectively. The majority of patients with and without GI-related events reported on the MOGISS had normal levels of vitamins B and homocysteine at baseline. Low levels (defined by less than the lower limit of normal) of folic acid at baseline were observed in 26.1% of patients without GI-related events and 30.2% of patients with GI-related events. Low levels of vitamin B12 at baseline were observed in 8.7% of patients without GI-related events and 17.7% of patients with GI-related events. Additional ad hoc analyses exploring the associations between levels of vitamins B at baseline and the presence of GI-related events using logistic regression revealed no statistically significant associations.
Discussion
In general, the frequency of GI-related events observed in TOLERATE was higher than in the placebo-controlled pivotal trials, where 27% and 38% of all DMF-treated patients experienced GI events within the first 3 months of treatment.8,9 This may be due to more strict enrolment criteria in the phase III trials (i.e. narrower age range, lower severity of MS disease as measured by the Expanded Disability Status Scale and exclusion of other clinically significant illness and prespecified laboratory abnormalities). Additionally, GI-related events were recorded by investigators at monthly visits based on retrospective reports by patients. In contrast, TOLERATE patients reported their GI status directly three times per day to an eDiary device using two numerical rating scales (MOGISS, MAGISS). This prospective method prompted self reporting, which potentially led to increased frequency, but also, accuracy of event reporting.11
Across all therapies, patients took symptomatic medications for a median of 5 days (range 1–85 days). Few patients continued to use symptomatic therapy by week 12; however, for some patients, GI symptoms persisted and required continuing medication use for symptomatic relief.
As a consequence of the higher rate of GI-related events in TOLERATE, the proportion of patients using symptomatic therapy was also higher (40%) than in the pivotal phase III studies (11–16%).
Serious adverse events and adverse events leading to discontinuation of study treatment were infrequent, despite the relatively high incidence of eDiary-reported GI-related events. These findings are consistent with the findings of the MANAGE study and suggest that GI-related events associated with DMF treatment are generally mild and not bothersome enough to lead to treatment interruption11. Discontinuation rates in TOLERATE due to GI-related events (6.6%) were consistent with the rates reported in MANAGE (7.2%).
As in the MANAGE study, taking DMF with food was recommended by an expert panel13 and is also part of the German product label. Patients were instructed to take DMF with or within 1 h of a meal, but not all patients regularly did so. In fact, less than a quarter (24%) of patients actually took DMF with food. A lower proportion of patients with GI-related events, 21%, reported always taking DMF with food compared with 48% of those who did not have GI events. The results of this study suggest that taking DMF with food may reduce the occurrence of GI events, especially in severe outcome of GI events according to the MOGISS. It may be hypothesized that the need for GI-symptomatic therapies could be eclipsed by counselling patients about the importance taking DMF with a meal. Additional analyses of larger sample sizes are warranted to further understand the association between taking DMF with food, especially with consideration of its composition (e.g. fatty foods) and the onset of GI events (Figure 3).
Among the 211 patients in the safety population, a range of 8.5–12.8% did not titrate fully to the 240 mg. This may be due to the recommendations of the MS guideline group in Germany (KKNMS) that recommended direct titrating over a period of up to 4 weeks immediately after the start of this study in order to reach the recommended dose of 240 mg to minimize GI-related adverse events16. Nevertheless, the adherence to protocol can be considered as high.
This study also investigated potential prognostic biomarkers for GI intolerance in a proof-of-concept approach. GI intolerance may develop as a consequence of a decreased ability to metabolise potentially irritating byproducts of DMF, such as methanol and formic acid.14 The percentage of patients with low baseline levels of folic acid and vitamin B12 was slightly higher in those experiencing GI-related events, although this difference never reached statistical significance. Further controlled studies are required to explore the hypothesis that patients with higher serum levels of B vitamins, which are required for methyl group metabolism, may experience fewer GI symptoms.
Most of the primary findings of TOLERATE were similar to those of MANAGE, in which 88% of patients reported GI-related events.13 In the MANAGE study, the frequency and severity of GI events also peaked in week 3 and declined thereafter among those who took symptomatic therapy. In both the TOLERATE and the MANAGE studies, approximately 7% of patients discontinued DMF due to GI-related AEs. Notably, no GI-related SAEs were reported in TOLERATE, whereas four were noted in the MANAGE study.
Several limitations may have influenced the results of this study. Symptoms were self reported and while incorporating that process, may have led to some benefits (i.e. higher frequency of reporting); the subjective descriptions of events provided were not reviewed or adjudicated by medical staff. This may have led to inaccurate or inconsistent reporting of events across all patients. Another potential limitation was that GI event data were restricted to the first 12 weeks of study treatment, which was intended to capture the initial and most pivotal reactions to DMF, as noted in the first 3 months of the phase III trials. Thus, the study design did not allow for the evaluation of the long-term effects of DMF on GI-related events and whether more patients may discontinue due to GI-related events beyond the examined study period12. Additionally, it is hypothetical to predict if decrease of GI symptoms would have occurred regardless of whether symptomatic GI therapies were taken as this approach would demand a nontreated control group. Finally, it should be noted that this study was conducted in Germany only, so the results may not be generalizable to the broader global population of patients taking DMF. However, the results of this study are consistent with those reported in the MANAGE study of patients in the US, and the proportions of reported GI symptom types, specifically abdominal pain, diarrhoea and vomiting, are comparable to those reported in patients taking DMF in routine care in the US.15 In addition, the timing of the majority of symptoms and the highest frequency of symptomatic therapy use were similar in both studies. The slightly higher proportion of symptomatic therapy use in the MANAGE as compared with the TOLERATE study may be attributable to different prescribing patterns across regions, as evidenced by differences in the types of symptomatic therapies used in these two studies.
In summary, GI events were among the most frequently reported AEs during this study, consistent with the known safety profile of the product as described in the prescribing information. Symptom frequency and severity peaked by the third week after starting DMF and then decreased to the end of the study at 12 weeks. Use of GI-related symptomatic therapy peaked within the first month and decreased through 12 weeks. There is some evidence that consistently taking DMF with food may help mitigate GI events that occur in patients taking DMF, though future research may further clarify the effects of food consumption on DMF-related GI events.
The results of TOLERATE indicate that gastrointestinal events associated with delayed-release DMF in routine clinical care are mainly mild to moderate in severity and can be managed with symptomatic therapies. Further, dosing with food and the use of symptomatic therapy may help to mitigate these events.
Supplemental Material
Supplemental material, Supplement_for_TOLERATE_manuscript for Incidence and mitigation of gastrointestinal events in patients with relapsing–remitting multiple sclerosis receiving delayed-release dimethyl fumarate: a German phase IV study (TOLERATE) by Ralf Gold, Eugen Schlegel, Birte Elias-Hamp, Christian Albert, Stephan Schmidt, Björn Tackenberg, James Xiao, Tom Schaak and Hans Christian Salmen in Therapeutic Advances in Neurological Disorders
Acknowledgments
Authors would like to thank all patients for voluntary participation.
Footnotes
Funding: This study, as well as medical writing support in the development of the manuscript, was funded by Biogen/Germany. The authors had full editorial control of the manuscript and provided their final approval.
Conflict of interest statement: RG received speaker’s and board honoraria from Baxter, Bayer Schering, Biogen, CLB Behring, Genzyme, Merck Serono, Novartis, Talecris, Teva and Wyeth. His department received grant support from Bayer Schering, Biogen, Genzyme, Merck Serono, Novartis and Teva. ES received travel expenses and grants from Biogen, Novartis, Roche, Icon and Takeda. BEH received research support and personal compensation for activities with Biogen, Merck Serono, Bayer Healthcare, Almirall, Novartis, Teva and Genzyme as a speaker. CA received travel/congress grants from Biogen, Novartis, Teva and Genzyme. SS received travel expenses, grants and honoraria from Biogen, Merck, Bayer Vital, Novartis, Genzyme, Roche and Teva. BT received speaker and consultancy honoraria as well as research grants and travel reimbursements from Bayer Healthcare, Biogen, CSL Behring, GRIFOLS, Merck Serono, Novartis, Octapharma, Roche, Sanofi Genzyme and Teva. JX, TS and HCS are employees of Biogen.
RG receives lecturing honoraria and advisory boards’ honoraria from Biogen. His institution got IIT funding from Biogen.
Supplemental Material: Supplementary material for this article is available online.
Contributor Information
Ralf Gold, Katholisches Klinikum Bochum St. Josef-Hospital, Neurological Clinic, Bochum University, Gudrunstraße 56, 44791 Bochum, Germany.
Eugen Schlegel, Centre for Neurological Studies, Siegen, Germany.
Birte Elias-Hamp, Institute for Clinical Studies, Hamburg, Germany.
Christian Albert, Neurological Clinic, St. Josef Hospital, Potsdam, Germany.
Stephan Schmidt, Neurological Study Centre, Bonn, Germany.
Björn Tackenberg, Department of Neurology, Marburg University, Marburg, Germany.
James Xiao, Biogen, Cambridge, MA, USA.
Tom Schaak, Biogen, Ismaning, Germany.
Hans Christian Salmen, Biogen, Ismaning, Germany.
References
- 1. Tullman MJ. A review of current and emerging therapeutic strategies in multiple sclerosis. Am J Manag Care 2013; 19(2 Suppl): S21–S27. [PubMed] [Google Scholar]
- 2. Farber RS, Sand IK. Optimizing the initial choice and timing of therapy in relapsing-remitting multiple sclerosis. Ther Adv Neurol Disord 2015; 8: 212–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perumal J, Khan O. Emerging disease-modifying therapies in multiple sclerosis. Curr Treat Options Neurol 2012; 14: 256–263. [DOI] [PubMed] [Google Scholar]
- 4. Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011; 134: 678–692. [DOI] [PubMed] [Google Scholar]
- 5. Scannevin RH, Chollate S, Jung MY, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther 2012; 341: 274–284. [DOI] [PubMed] [Google Scholar]
- 6. Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol 2004; 251: 261–268. [DOI] [PubMed] [Google Scholar]
- 7. Kappos L, Gold R, Miller DH, et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 2008; 372: 1463–1472. [DOI] [PubMed] [Google Scholar]
- 8. Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 9. Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 10. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fox EJ, Vasquez A, Grainger W, et al. Gastrointestinal tolerability of delayed-release dimethyl fumarate in a multicenter, open-label study of patients with relapsing forms of multiple sclerosis (MANAGE). Int J MS Care 2016; 18: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miclea A, Leussink VI, Hartung HP, et al. Safety and efficacy of dimethyl fumarate in multiple sclerosis: a multi-center observational study. J Neurol 2016; 263: 1626–1632. [DOI] [PubMed] [Google Scholar]
- 13. Phillips JT, Hutchinson M, Fox R, et al. Managing flushing and gastrointestinal events associated with delayed-release dimethyl fumarate: experiences of an international panel. Mult Scler Relat Disord 2014; 3: 513–519. [DOI] [PubMed] [Google Scholar]
- 14. Liesivuori J, Savolainen H. Methanol and formic acid toxicity: biochemical mechanisms. Pharmacol Toxicol 1991; 69: 157–163. [DOI] [PubMed] [Google Scholar]
- 15. Wicks P, Rasouliyan L, Katic B, et al. The real-world patient experience of fingolimod and dimethyl fumarate for multiple sclerosis. BMC Res Notes 2016; 9: 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gold R, Hohlfeld R, Meyer U. Qualitätshandbuch Multiple Sklerose - Empfehlungen zur Therapie der MS für Ärzte. Krankheitsbezogenes Kompetenznetz Multiple Sklerose e.V.- Ausgabe 2017; 39-54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplement_for_TOLERATE_manuscript for Incidence and mitigation of gastrointestinal events in patients with relapsing–remitting multiple sclerosis receiving delayed-release dimethyl fumarate: a German phase IV study (TOLERATE) by Ralf Gold, Eugen Schlegel, Birte Elias-Hamp, Christian Albert, Stephan Schmidt, Björn Tackenberg, James Xiao, Tom Schaak and Hans Christian Salmen in Therapeutic Advances in Neurological Disorders