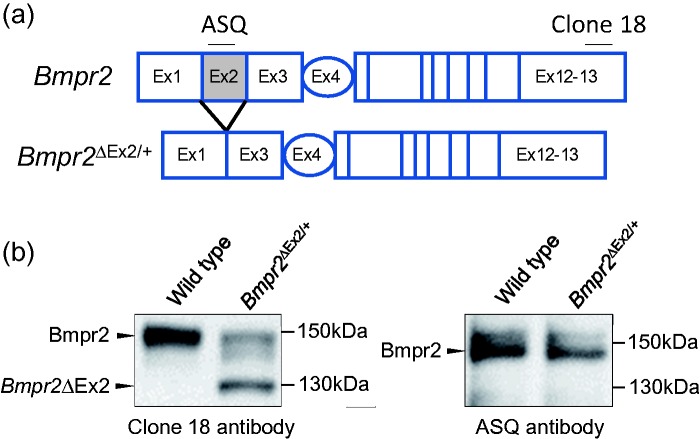
Fig. 1.
Characterization of the Bmpr2ΔEx2 mutant protein product in PECs from Bmpr2ΔEx2/+ mice. (a) Schematic representation of the Bmpr2 gene. Exons 1–3 encode the extracellular domain of Bmpr2; exon 4 encodes most of the transmembrane domain; exons 5–13 encode the intracellular kinase domain and C-terminal tail. In-frame deletion of Exon 2 in Bmpr2ΔEx2/+ mutant mice, and corresponding amino-acid sequences recognized by the anti-BMPR2 antibodies clone 18 and ASQ, are indicated. (b) Conditionally immortalized PECs (ciPECs) were isolated from WT control and Bmpr2ΔEx2/+ mice. These cells were generated, as previously described,18,37,88 by crossing WT or Bmpr2 mutant mice on a C57Bl/6 background with C57Bl/6 H-2Kb-tsA58 SV40 large T antigen transgenic mice (Charles River's “Immortomouse”). Primary PECs were isolated from macerated mouse lungs perfused with trypsin and selected using microvascular endothelial cell medium under permissive conditions (in the presence of Interferon-γ at 33℃). For experiments, cells were transferred to 37℃ without Interferon-γ for 3–5 days to inhibit SV40 large T antigen activity, enabling the cells to become quiescent and differentiate into polarized PECs.18,37,88 Western blot using mouse monoclonal Clone 18 (BD) and ASQ (in house) anti-BMPR2 antibodies in WT and Bmpr2ΔEx2/+ ciPEC lysates, as described.18 Both cell lines expressed a 150-kDa WT Bmpr2 product which is detected using both antibodies. Bmpr2ΔEx2/+ ciPECs also express a 130-kDa product that is detected with Clone 18, which recognizes a C-terminal peptide sequence that is preserved in both WT Bmpr2 and in the Bmpr2ΔEx2 mutant product, but not the ASQ antibody, which recognizes an amino-acid sequence encoded by exon 2. These western blots have not previously been published but similar studies have been published in Frump et al.18