Figure 4.
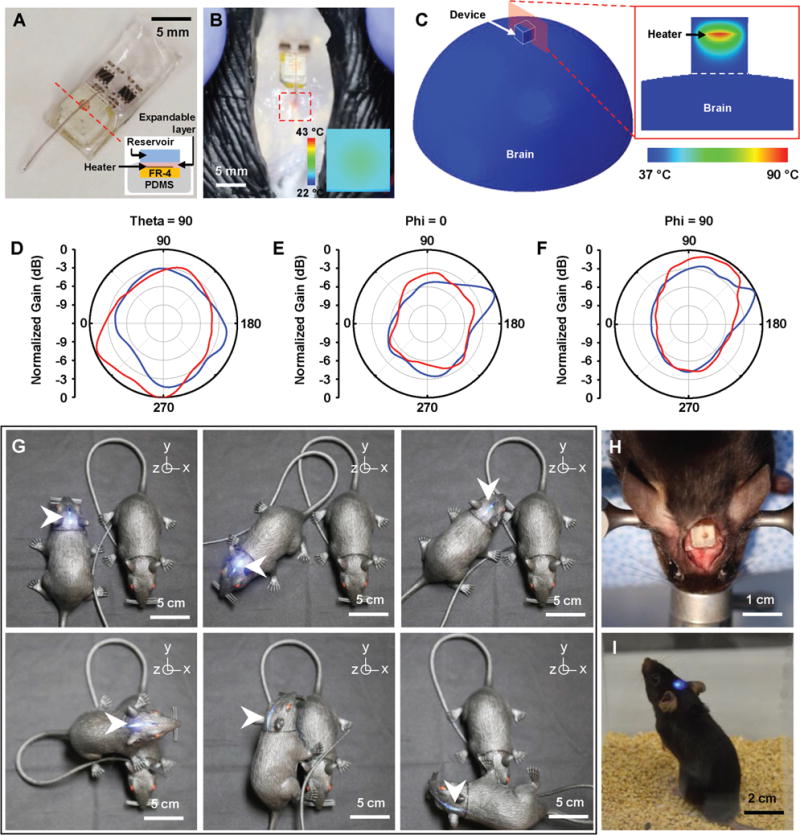
Feasibility study of fully implantable, battery-free, wireless optofluidic systems for operation in freely moving animals. A) Optical image of a wireless optofluidic device encapsulated with 2 mm thick PDMS for thermally safe operation of the heater on brain tissue. The inset shows the cross-sectional image of the device, defined by the red dotted line. B) Wireless delivery of red dye into the phantom brain (0.6% agarose gel) in the rat model. The red dotted box indicates the infused dye. IR image in the inset shows surface temperature of the heater’s PDMS encapsulant (≈30 °C) in ambient environment at room temperature when the heater temperature reaches ≈100 °C during fluid delivery, verifying rapid heat dissipation through the PDMS encapsulant. C) FEA modeling of temperature distribution at the surface of the 3D model simulating the device operated on the brain surface (left) and that at the cross-section cut by the red plane (right). D–F) Normalized angular radiation pattern of two-channel antennas in optofluidic systems. Cross-sectional view at D) θ = 90°, where polar angle θ is the angle measured from the zenith direction of the antenna plane, E) φ = 0°, where azimuthal angle φ is the angle measured from the orthogonal projection onto the antenna plane from the direction toward the antenna input, and F) φ = 90°. G) Pictures of a model rat implanted with a wireless optofluidic system, demonstrating wireless operation at various angles. H) Optical image showing full implantation of an optofluidic device into a mouse’s head. I) Optical image of a freely behaving mouse with an optofluidic device implanted under the skin, on the skull.
