Abstract
The protocol adopted in this work aims at unraveling how X-rays perturb the functioning of the intestinal barrier, focusing on the interplay between colorectal tumor cells and the immune system. Colorectal carcinoma is among the most common type of cancer, typically treated by surgery, chemotherapy, and radiotherapy. Advantages of radiotherapy in targeting the tumor are well known. However, even limited exposures of healthy tissues are of great concern, particularly regarding the effects on the intestinal barrier and the immune system. The adopted setup allows to study the interplay between two cell populations in a condition more similar to the physiological one, when compared to normal cell cultures. For this purpose, we resort to different techniques and we used an in vitro co-culture model, based on Caco-2 cells differentiated as a monolayer and PBMC, sharing the same culture medium. This protocol has been developed to focus on both macroscopic effects, i.e. cell viability and Trans-Epithelial Electrical Resistance (TEER), and, through western blot, molecular alterations, i.e. the activation of inflammatory pathway in immune cells and the tight junction protein expression in Caco-2 cells. Initial evaluation of radiation effects on Caco-2 cell viability was assessed via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Trypan blue assays, while TEER was measured at fixed time intervals through an ohmmeter specifically designed for co-culture systems. In this way, the effects due to radiation, the presence of Peripheral Blood Mononuclear Cells (PBMC), and eventually their synergistic effect, can be demonstrated. Through these complementary techniques, we observed a high radio-resistance of Caco-2 within the range of 2 - 10 Gy of X-rays and an increased Caco-2 monolayer permeability when PBMCs were added. In particular, PBMC presence was found to be associated with the variation in the tight junction scaffold proteins expression.
Keywords: Immunology, Issue 131, X-rays, ionizing radiation, Caco-2, colorectal carcinoma, Trans-Epithelial Electrical Resistance, western blotting, co-culture, peripheral blood mononuclear cells, tight junctions, immunotherapy, radiotherapy
Introduction
The methodology adopted in this work was designed to investigate the interplay between colorectal cancer cells and the immune system, exploiting a set-up closer to the physiological condition when compared to normal 2-dimensional cell cultures.
Colorectal carcinoma (CRC) is considered the third most frequent type of cancer, with more than one million of cases worldwide (Global Cancer Observatory, International Agency for Research on Cancer, World Health Organization, http://gco.iarc.fr). Management of CRC is routinely performed through either surgery, chemotherapy or radiotherapy1. When compared to invasive techniques like surgery or chemotherapy, radiotherapy largely avoids the typical detrimental systemic reactions deriving from these clinical approaches, thanks to the localized delivery of radiation dose. However, side-effects can arise in the surrounding healthy tissues, triggering inflammation with direct damage to healthy cells and damage mediated by non-targeted effects2,3,4. Focusing on the adverse effects due to the irradiation during colorectal cancer treatment, two aspects need to be investigated. First, the mechanisms responsible of intestinal impermeability could be altered by radiation delivery causing, in turn, the possibility of side effects due to an altered containment of bacterial population and the paracellular passage of molecules and solutes. Second, the presence of gut-associated lymphatic tissue (GALT) acts as an outpost of the immune system, with the function of controlling bacterial growth and mediating the general immune response5,6,7. To fulfill these functions, intestinal impermeability is kept due to the function of junctional complexes between cells' membranes. For these reasons, the induced detrimental consequences due to different doses of X-rays were investigated in Caco-2 cells alone and in co-cultures with PBMC.
Although conducting studies on cell cultures is the first setline of investigation in biomedical research, the lack of detailed knowledge of the mechanisms driving cell biology and reciprocal interactions between different cell types might become critical when approaching the study of physiology of organs, systems, and apparatuses that cannot be easily recreated in the laboratory. Therefore, we decided to adopt a co-culture set-up, allowing both the study of two cell populations together and the dissection of aspects related to intercellular and extracellular mechanisms.
Co-culture is a technique exploited when studying epithelial functions and the interplay between different cell types. In particular, the use of such a technique becomes mandatory in our case, because epithelia are made up of cells characterized by polarity. In the case of the intestinal barrier, enterocytes show a well-defined polarization, with apical and basolateral poles normally separated due to the presence of tight junction-creating adhesion molecules. This compartmentalization is needed for the tissue physiology, avoiding paracellular trafficking and allowing the passage of determined molecules only. This feature is of course impossible to recreate with a normal cell culturing set-up. Moreover, the adoption of the co-culture set-up reproduces the presence of immune cells only in the basolateral surface, while the apical surface (corresponding to intestinal lumen) is not directly in contact with other cells.
Recently, Caco-2 cell lines gained more importance as an in vitro model of intestinal barrier. Although derived from human colon adenocarcinoma, Caco-2 cells maintain the differentiation ability and create a functional polarized monolayer8, which allows the investigation of cell membrane properties when grown in a co-culture insert.
Since Caco-2 culturing on a porous membrane is a well-established in vitro model of intestinal monolayer, an improvement has been the co-culture between Caco-2 and other cells. This set-up has been adopted frequently to measure the crosstalk between different cell types9 and can be used to unravel Caco-2 perturbed response to exogenous stimuli when in co-culture, with respect to Caco-2 cultured alone.
Many studies have addressed Caco-2 behavior when co-cultured with both non-pathogenic bacteria and peripheral blood mononuclear cells, to elucidate in particular the crosstalk with the immune system10. Pozo-Rubio et al.11 studied the expression of several cytokines in a Caco-2/PBMC co-culture with bifidobacteria stimulating Caco-2 cells. Their work highlighted substantial modification to cytokine expression profiles depending on bacterial stimulation performed in presence/absence of PBMC. Their results lead to the conclusion that the presence of PBMC sensitizes Caco-2 to bifidobacteria.
Different responses of Caco-2 cells to non-pathogenic and pathogenic bacteria have been assessed by different research teams. Parlesak et al.12 demonstrated the immunosuppressive effects of Caco-2 cells on Escherichia coli-stimulated PBMC. Moreover, Haller et al.13 studied the response of Caco-2 cells stimulated with both lipopolysaccharide (LPS) from enteropathogenic E. coli spp. or non-enteropathogenic bacteria i.e.E. coli spp., Lactobacillus spp., strengthening the conjecture that the Caco-2 cells' response strictly depends on the presence of leukocytes in the co-culture set-up.
By performing different complementary laboratory assays (e.g. western blot, trans-epithelial electrical resistance, MTT, etc.), in addition to the analysis of different cell types grown in co-culture, the whole methodology promises results that can be considered more representative of what really happens in vivo. Moreover, this set-up enables the separation of the different co-culture compartments, allowing not only the study of the cell types involved but also the intercellular signaling molecules released in the upper vs. lower compartment or in presence vs. absence of co-culture.
Protocol
The following protocol involves human blood withdrawal from healthy volunteers. Donors provided written informed consent prior to enrollment. This procedure is in accordance with the Helsinki Declaration and blood withdrawals were performed by a professional healthcare assistant.
1. Cell Culture and Co-culture Set-up
One week before the irradiation, prepare a Caco-2 cell suspension containing 2.5 × 105 cells/mL in fresh RPMI1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Seed 2 mL of cell suspension in sterile 1 µm-pore diameter cell culture inserts for 6-well plates and put the insert into a 6-well plate. NOTE: Cell culture inserts might need to be activated by incubation with sterile complete medium prior to cell seeding. In this case, culture media should be discarded and replaced with cell suspension media.
Add 3 mL of fresh RPMI1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 IU/mL penicillin, and 100 µg/mL streptomycin in each bottom compartment, and culture the cells at 37 °C in an incubator with humidified atmosphere containing 5% CO2.
On the same day of Caco-2 cell irradiation, collect human whole blood in commercially available lithium-heparin coated 6 mL tubes (tube size: 13 x 100 mm).
Subsequently, isolate peripheral blood mononuclear cells (PBMC) by using Ficoll gradient. To separate PBMC, put 25 mL of Ficoll in a 50 mL conical centrifuge tube and layer an equal volume of whole blood diluted 1:1 with RPMI1640 onto the Ficoll surface. NOTE: A normal healthy donor usually has approximately 4 - 10 × 106 PBMC/mL.
Centrifuge the 50 mL tubes at 400 x g for 30 min at room temperature.
Gently collect the PBMC at the interface between Ficoll and plasma by aspiration with a Pasteur pipette and put them in a 15 mL conical tube.
Wash the PBMC twice by adding 10 mL of phosphate buffered saline (PBS) and centrifuging PBMC at 250 x g for 10 min.
Culture PBMC for a maximum of 3 - 5 h in T25 cm2 flasks in complete RPMI1640 media, as described before, at 37 °C in a humidified atmosphere containing 5% CO2. NOTE: Collect PBMC on the day of the experiment and seed 2 × 106 cells/well, suspended in 3 mL of complete RPMI1640 medium, in the bottom compartment of the co-culture. Inserts with Caco-2 cells are transferred in PBMC-containing wells 30 min after their irradiation.PBMC collected from whole blood cannot be maintained in culture for more than approximately 72 h, if not stimulated with e.g. phytohaemagglutinin (PHA), after which cells' viability is lost. CAUTION: The quality and safety of all blood and blood products must be guaranteed throughout the whole process.
2. Irradiation Set-up
Note: Irradiation of Caco-2 cells was performed at the radiotherapy department of Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) S. Maugeri (Pavia, Italy) with a linear accelerator routinely used to treat different types of cancers.
Set photon X-rays energy to 6 MV peak. The linear accelerator operates in a pulsing regime (time between two consecutive pulses approximately of 4 ms; duration of a single pulse about 5 µs with a dose rate up to 1 × 10-3 Gy/p).
Place the 6-well plates containing inserts with Caco-2 on the trajectory of X-rays on a 1.4 cm-thick Plexiglas sheet (corresponding to a distance slightly greater than the build-up of the used radiation) at 100 cm from the source of radiation.
Place a 0.5 cm-thick bolus on each sample before the irradiation occurs to guarantee the backscattered radiation component and the charged particles equilibrium.
Irradiate the cells (doses in the range 2 - 10 Gy) with a flat and symmetric (± 2%) 20 x 20 cm2 radiation field and a dose-rate of 3 Gy/min. NOTE: Control "sham"-irradiated cells underwent the same procedural conditions of the irradiated ones, without entering the irradiation room (received dose: 0 Gy). CAUTION: Ionizing radiation-producing devices must be used only by specialized personnel.
3. Cell Viability Assay (MTT Assay)
Note: Caco-2 cell metabolic activity was assessed through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay14.
Seed 2 × 105 Caco-2 in a 24-well plate 24 h before irradiation in 1.25 mL of complete medium.
Irradiate the cells as described in steps 2.1 - 2.4 then incubate the cells at 37 °C in a humidified atmosphere containing 5% CO2.
21 h after irradiation, add 100 µL of 5 mg/mL MTT solution and keep the cells at 37 °C in a humidified atmosphere containing 5% CO2 for 3 h.
Discard the supernatant and wash the cells with 1 mL of PBS.
Dissolve formazan crystals by adding 500 µL of dimethyl sulfoxide (DMSO) in each well and evaluate the absorbance with a multi-well plate reader at λ = 570 nm.
Repeat step 3.3 - 3.4 - 3.5 - 3.6 at 45 h after irradiation. NOTE: Results are shown as a perturbation from the corresponding sham condition, which is normalized to 100%. CAUTION: DMSO is inflammable and irritating agent for skin, eyes and respiratory system (GHS07, GHS08). In case of contact with eyes, wash immediately with abundant water and seek medical advice. MTT is an irritating agent for skin, eyes and respiratory system (GHS07, GHS08). In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
4. Percentage of Viable Cells Determination (Trypan Blue Dye Exclusion Assay)
NOTE: Percentage of viable cells was assessed by Trypan Blue Dye Exclusion assay.
Seed 2 × 105 Caco-2 in 24-well plate 24 h before irradiation in 1.25 mL of complete medium.
Irradiate the cells with doses in the range 0 - 10 Gy (see steps 2.1 - 2.4) then incubate the cells at 37 °C in a humidified atmosphere containing 5% CO2 for 24 - 48 h.
After the two chosen time points, wash the cells with PBS, discard it, then add 100 µL of Trypsin-ethylenediaminetetraacetic acid (EDTA) solution. Put the cells at 37 °C in a humidified atmosphere containing 5% CO2 for 2 min then add complete medium to arrest the enzymatic reaction.
Collect the cells in a 1.5 mL-tube and centrifuge the tube at 500 x g for 5 min. Discard the supernatant and re-suspend the cells in 50 µL of PBS.
Mix the cell suspension with 50 µL of Trypan Blue dye solution and incubate the mixture for 3 min at room temperature.
Count the stained (non-viable) and unstained (viable) cells with a Bürker chamber.
5. Trans-Epithelial Electrical Resistance (TEER)
Seed 5 × 105 Caco-2 cells one week prior to irradiation in 6-well plate co-culture inserts (polyethylene terephthalate (PET), 2 x 106 pores/cm2).
1 h before irradiation, measure the TEER with a voltmeter/ohmmeter.
Irradiate cells as described in steps 2.1 - 2.4.
Incubate Caco-2 cells in presence/absence of co-culture with PBMC at 37 °C in a humidified atmosphere containing 5% CO2.
For the first few hours post-irradiation, measure TEER every hour, and subsequently every 3 h up to 48 h.
6. Western Blot Analysis of Claudin-1, Occludin, Afadin, ZO-1, ZO-2, NF-kB, and XIAP
Seed 5 × 105 cells 1 week before X-rays exposure in 6-well plate co-culture inserts (PET, 2 x 106 pores/cm2) and irradiate cells as described in steps 2.1 - 2.4.
Incubate Caco-2 cells in presence/absence of co-culture with PBMC at 37 °C in a humidified atmosphere containing 5% CO2.
48 h after irradiation, prepare Caco-2 and PBMC cell lysates by treating cells with cell lysis buffer and store samples at -20 °C. NOTE: the protocol can be paused here.
Quantify total protein amount by bicinchoninic acid (BCA) method. NOTE: the protocol can be paused here.
Mix equal amounts of total proteins with Laemli Sample Buffer added with β-mercaptoethanol (final concentration of β-mercaptoethanol is 5%), heat the samples at 95 °C for 5 min and spin them at 10,000 x g.
Load samples in a 4 - 20% precast gel and perform the electrophoresis at 120 V for 1 h. Transfer proteins on a polyvinildiene fluoride (PVDF) membrane.
Block non-specific binding sites by incubating PVDF membrane at room temperature with 5% non-fat dry milk (NFDM) in PBS added with 0.2% Tween-20 (PBT). Wash the membrane three times with 10 mL of 0.2% PBT for 5 min.
Incubate the membrane with gentle agitation for 1 h at room temperature prior to being placed overnight at 4 °C with the following primary antibodies: anti-claudin-1, anti-ZO-1, anti-ZO-2, anti-afadin, anti-occludin, anti-NF-kB, anti-XIAP, and anti-actin. Wash the membrane three times with 10 mL of 0.2% PBT for 5 min.
Incubate the membranes with Horseradish Peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature with gentle agitation. Wash the membrane three times with 10 mL of 0.2% PBT for 5 min.
At room temperature, develop photographic films by incubating the membranes with enhanced chemo-luminescent kit.
Acquire photographic films with a scanning system and quantify the obtained bands with suitable image analysis software. NOTE: The evaluation of Claudin-1, Occludin, Afadin, ZO-1, and ZO-2 is performed in Caco-2 cells. The evaluation of NF-kB and XIAP is performed in PBMC. CAUTION: β-mercaptoethanol is toxic (GHS05, GHS06, GHS08, GHS09). Do not breathe vapors, avoid release into the environment, and wear individual protection devices and respiratory protection. If swallowed, immediately ask for medical advice.
7. Statistical analysis
To determine whether the radiation exposure and the co-culture induce a statistically significant perturbation, perform a two-way analysis of variance (ANOVA) test with multiple comparisons for repeated measurements (with Bonferroni post-hoc tests to compare replicate means).
If not otherwise stated, calculate the statistical significance (p) by two-tailed Student's t-test. Each value is the mean of ≥3 independent experiments ± Standard Error of the Mean (SEM).
Representative Results
One week prior to the experiment, cells are sown onto the porous membrane of the insert and allowed to grow during the following days. The level of the confluence can be checked either using an inverted microscope or via the measurement of the TEER values. Indeed, during the growth phase, TEER keeps increasing until all the porous membrane has been covered by cells and they form a differentiated cell monolayer. If cells proliferate faster/slower, the experiment could start at earlier/later time points after being seeded. When at confluence, cells are then brought to the irradiation facility, minimizing the imparted environmental stress (temperature or pH), prior to starting the co-culture with/without PBMC, seeded in the bottom compartment (Figure 1A), or evaluating the proliferation of Caco-2 cells. Given the initial seeding density, on day 0 cells should reach 100% confluence and create a differentiated monolayer of epithelial cells, which can be observed by the plateau in TEER shown in Figure 1B. Once the cells reach such status, the TEER value is kept relatively constant over the following week, as long as the old culture medium is replaced with fresh medium, at least once a week (Figure 1B). As shown in Figure 1C, the MTT assay does not show any statistically significant alteration of the proliferative status of Caco-2 cells neither at 24 h nor at 48 h, independently of the dose received (up to 10 Gy).
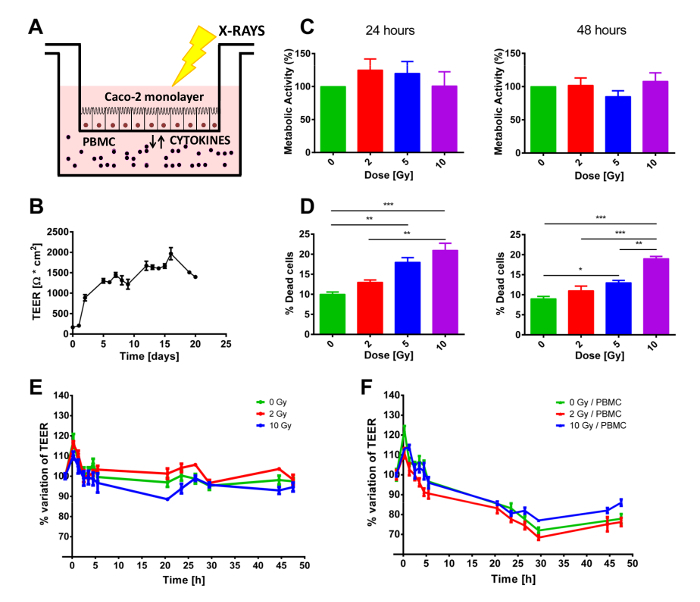
A different result was observed concerning the short-term mortality of Caco-2 cells. At both time points, Trypan blue staining show a dose-dependent increase in cell mortality. These results show a clear effect of the radiation exposure, although the percentages of dead cells appear to be very low, particularly when considering that the highest delivered dose (10 Gy) produces only roughly 20% of cell death (Figure 1D).
Samples were co-cultured with or without PBMC in the lower compartment. Given the fact that PBMC did not receive any external stimulus to proliferate, a 48 h experiment was considered ideal to avoid the bias introduced by PBMC starting to die. Therefore, from immediately before the irradiation, TEER was regularly measured for 48 h, to keep track of possible transitory effects caused by the irradiation protocol. As shown in Figure 1 E-F, TEER values are presented as relative variations with respect to the pre-treated condition (which were of the order of 1200 - 1500 Ω·cm2) to better highlight the perturbation induced by the X-ray irradiation and/or by the presence/absence of PBMC in the co-culture. In both cases, an initial transient peak can be clearly seen at the first time point after the radiation exposure, which can be attributed to the irradiation procedure.
When not in co-culture with PBMC (Figure 1E), TEER values are almost constant up to 48 h while, after 10 Gy of X-rays, cells show a prolonged decrease in TEER beginning at 3 h post-irradiation. The presence of PBMCs completely modifies the TEER temporal dynamics (Figure 1F). For all doses, a reduction in the TEER is clearly observable from 3 h up to approximately 30 h post-irradiation, when TEER appears to settle at a constant value (Figure 1F).
Tight junction complexes expression levels were investigated in Caco-2 cells lysates through western blot assay. Caco-2 cells were exposed to ionizing radiation (with doses of 0, 2, and 10 Gy) and subsequently grown alone or in co-culture with PBMCs in the bottom compartment for 48 h (as shown in Figure 2A-F). Both Claudin-1 and Occludin (Figure 2A, 2B) were found to be not significantly altered by X-ray and/or co-culture with PBMC. Large fluctuations were instead observed in scaffold proteins ZO-1, ZO-2 and Afadin (Figure 2C, 2D, 2E). In particular, a reduction in ZO-2 expression levels is observed already after 2 Gy when in co-culture with PBMC while only at 10 Gy when Caco-2 were growing alone. Afadin expression levels instead are affected only after 10 Gy of X-rays, with an additional reduction when Caco-2 are co-cultured with PBMCs.
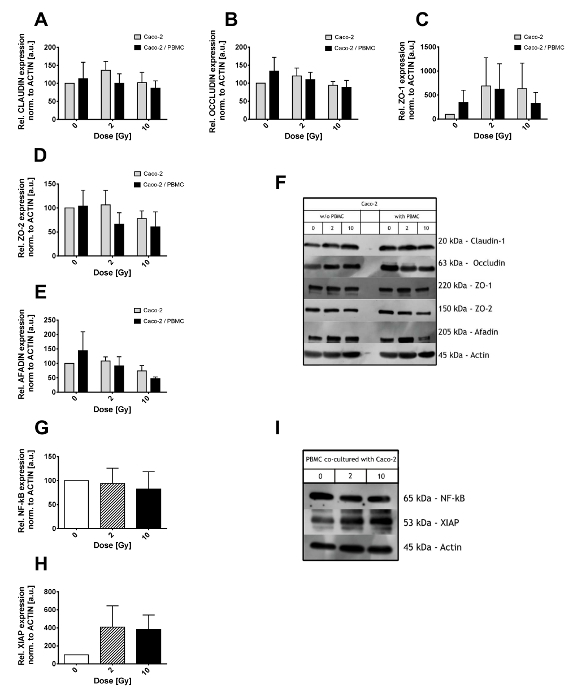
PBMCs co-cultured with Caco-2 were analyzed regarding the inflammatory state, in particular, the Nuclear Transcription Factor kB (NF-kB) and the X-linked inhibitor of apoptosis protein (XIAP) levels have been investigated (Figure 2 G-I). NF-kB total amount was not affected by the co-culture with Caco-2 exposed to different radiation doses (Figure 2G). On the contrary, XIAP levels were 4-fold up-regulated in both the 2 Gy and 10 Gy co-cultures, although the large variations demand a higher number of samples analyzed to reduce such fluctuations and gain a better statistical power.
As shown in Figure 2F and 2I, some non-specific bands might appear next to the expected molecular weight of the protein of interest. Unless true and non-specific bands are easily distinguishable, different antibody and/or BSA or NFDM concentrations should be considered.
Figure 1. Overall experimental setup and macroscopic effects of radiation exposure and/or PBMC co-culture. A) Schematic depiction of the co-culture model. B) TEER values measured daily from the initial seed of Caco-2 cells to assess the proper growth and differentiation status of the monolayer. C) Cell viability and D) mortality in Caco-2 exposed to X-rays (0, 2, 5, and 10 Gy). E) TEER measurements in Caco-2 cells irradiated with 0, 2, and 10 Gy of X-rays cultured without or F) with PBMCs. Each value is the mean of ≥3 independent experiments ± SEM. * p-val <0.05; ** p-va l< 0.01; *** p-val < 0.001. Graphs adapted from Morini et al.15. Please click here to view a larger version of this figure.
Figure 2. Western Blot results of Caco-2 and PBMC lysates. Expression level of the tight junction proteins (Claudin-1 (A), Occludin (B), ZO-1 (C), ZO-2 (D), and Afadin (E)) in Caco-2 after 0, 2, and 10 Gy of X-rays and in presence/absence of PBMC in co-culture. Values are normalized on Actin level. Illustrative films for each tight junction protein and conditions are shown in panel (F). Expression level of NF-κB (G) and XIAP (H) in PBMCs co-cultured with Caco-2 cells. Representative films for NF-kB, XIAP, and Actin are shown in panel. Each value is the mean of ≥3 independent experiments ± SEM. Graphs adapted from Morini et al.15. Please click here to view a larger version of this figure.
Discussion
Colorectal cancer, with its high occurrence in developed countries, is one of the most frequent causes of morbidity and mortality in the population. It is usually managed by surgery, chemotherapy, and radiotherapy1. In the framework of radiotherapy treatments, particular attention must be given to the effects of healthy tissue exposure4; moreover, systematic studies on the relationship between radiation exposure and the immune system are fundamental for developing approaches of radio-immunotherapy3.
The methodology we adopted in this work has been tailored to the investigation of Caco-2 cells and PBMC crosstalk. We focused on the effect of X-rays exposure of tumor cells, but the same protocol can be adapted to studies with pharmacological agents. Being closer to physiological conditions with respect to standard cell culturing, the strong advantage of this method is the possibility of a complete dissection of a complex system, given the possibility of analyzing both different cell types and the release of signal molecules into the two compartments of the co-culture itself. In this way, routinely applied biological methods can help the understanding of cellular interplay related mechanisms.
The initial characterization of X-ray-induced effects on Caco-2 cells was based on two complementary measurements, i.e. the MTT colorimetric assay and the Trypan blue dye exclusion test. The apparently inconsistent results could be explained by the different focus of these two assays. MTT assesses the oxidoreductase enzymes activity, while the Trypan blue dye is based on a living-cell exclusion dye mechanism.
The investigation of Caco-2-PBMC interplay requires the creation of an epithelial monolayer able to lead to a complete separation between the two compartments of the co-culture. The possibility of seeding Caco-2 cells in the co-culture insert allows the irradiation of only this cell population. Since the co-culture starts after irradiation, there is no bias due to any accidental exposure of PBMC. This set-up needs to be carefully handled to avoid damage (or contamination) to the cellular monolayer during the movements of the insert from one 6-well plate to the other. When carefully performed, TEER measurements are a simple and non-invasive method to investigate the monolayer permeability. This assay is strictly related to the co-culture set-up, and it is not the only choice for investigation of monolayer permeability. It allows a good reproducibility of measures once it is well calibrated with fresh complete medium. Common assays focus on the diffusion of chemical dyes from the upper "apical" compartment to the bottom "basolateral" one (e.g. fluorescein isothiocyanate (FITC)-dextran assay)16. However, since PBMC are present in the basolateral compartment in this study, we decided to avoid the introduction of chemical reagents in the experiments.
Among the different techniques adopted in this work, TEER measurement is the only one that requires the co-culture set-up17,18. However, the other common laboratory techniques provide more informative results when applied to cells in co-culture, as they allow the investigation of a setup closer to physiological conditions and the data have a stronger biological significance. On the other hand, it must be noted that the use of cells grown on a porous support could lead to some difficulties in the operations needed to prepare the samples, such as the cell lysis for the preparation of cellular extracts to be analyzed through western blotting.
The system adopted in this study has the potential to be further improved, e.g. with the application of substances able to recreate the extracellular matrix milieu. However, this will also result in an increased complexity of the system, and a complete dissection of the set-up will be more difficult to be achieved.
Setups for cell co-culture certainly represent a powerful tool for the advancement of in vitro research, and for the understanding of complex systems. This technique has the potential to increase the knowledge on fundamental mechanisms, providing new inputs to basic research studies on molecular signaling, and with possible applications to the modulation of the activity of the immune system in the framework of oncological clinical patient management.
Disclosures
The authors declare no conflict of interest.
Acknowledgments
Italian Institute for Nuclear Physics (INFN) partially funded this work through the INFN-MERIDIAN project. The authors acknowledge Prof. Edoardo Milotti (Physics Department, University of Trieste, Italy) for INFN-MERIDIAN project coordination; Dr. Roberto Chignola (Biotechnology Department, University of Verona, Italy) for providing the Caco-2 cells, the ohmmeter, and for his valuable help and training. We also acknowledge Agnese Solari for technical assistance.
References
- Cunningham D, et al. Colorectal cancer. Lancet. 2010;375(9719):1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- Mancuso M, et al. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. PNAS. 2008;105(34):12445–12450. doi: 10.1073/pnas.0804186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Formenti SC. Can abscopal effects of local radiotherapy be predicted by modeling T cell trafficking. J Immunother Cancer. 2016;4(1):29. doi: 10.1186/s40425-016-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott KR, et al. Biological mechanisms of normal tissue damage: Importance for the design of NTCP models. Radiother Oncol. 2012;105(1):79–85. doi: 10.1016/j.radonc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Derer A, Deloch L, Rubner Y, Fietkau R, Frey B, Gaipl US. Radio-immunotherapy-induced immunogenic cancer cells as basis for induction of systemic anti-tumor immune responses - pre-clinical evidence and ongoing clinical applications. Front Immunol. 2015;6:505. doi: 10.3389/fimmu.2015.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey B, Gaipl US. Radio-immunotherapy: The focused beam expands. Lancet Oncol. 2015;16(7):742–743. doi: 10.1016/S1470-2045(15)00055-8. [DOI] [PubMed] [Google Scholar]
- Prise KM, O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9(5):351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambuy Y, De Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. 2005;21(1):1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- Babini G, et al. Mechanisms of the induction of apoptosis mediated by radiation-induced cytokine release. Radiat Prot Dosim. 2015;166(1-4):165–169. doi: 10.1093/rpd/ncv133. [DOI] [PubMed] [Google Scholar]
- Pellegrina CD, et al. Effects of wheat germ agglutinin on human gastrointestinal epithelium: Insights from an experimental model of immune/epithelial cell interaction. Toxicol Appl Pharm. 2009;237(2):146–153. doi: 10.1016/j.taap.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Pozo-Rubio T, et al. Immunostimulatory effect of faecal Bifidobacterium species of breast-fed and formula-fed infants in a peripheral blood mononuclear cell/Caco-2 co-culture system. Brit J Nutr. 2011;106(8):1216–1223. doi: 10.1017/S0007114511001656. [DOI] [PubMed] [Google Scholar]
- Parlesak A, Haller D, Brinz S, Baeuerlein A, Bode C. Modulation of cytokine release by differentiated CACO-2 cells in a compartmentalized coculture model with mononuclear leucocytes and nonpathogenic bacteria. Scand J Immunol. 2004;60(5):477–485. doi: 10.1111/j.0300-9475.2004.01495.x. [DOI] [PubMed] [Google Scholar]
- Haller D. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut. 2000;47(1):79–87. doi: 10.1136/gut.47.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Morini J, Babini G, Barbieri S, Baiocco G, Ottolenghi A. The Interplay between Radioresistant Caco-2 Cells and the Immune System Increases Epithelial Layer Permeability and Alters Signaling Protein Spectrum. Front Immunol. 2017;8:1–12. doi: 10.3389/fimmu.2017.00223. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar S, Harms H, Runkler N, Maisner A, Kim KS, Schneider-Schaulies J. Measles virus-induced block of transendothelial migration of T lymphocytes and infection-mediated virus spread across endothelial cell barriers. J Virol. 2008;82(22):11273–11282. doi: 10.1128/JVI.00775-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, Hickman JJ. TEER Measurement Techniques for In Vitro Barrier Model Systems. J Lab Autom. 2015;20(2):107–126. doi: 10.1177/2211068214561025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes SM, Killick EM, Morris JF, Kadhim MA, Hill MA, Carr KE. Changes produced by external radiation in parameters influencing intestinal permeability and microparticle uptake in vitro. Int J Radiat Biol. 2008;84(6):467–486. doi: 10.1080/09553000802078388. [DOI] [PubMed] [Google Scholar]


