ABSTRACT
Background: Tick-borne pathogens (TBPs) are frequently studied in developed nations but are often neglected in emerging countries. In Mongolia, TBP research is especially sparse, with few research reports focusing upon human and domestic animal disease and tick ecology. However, little information exists on TBPs in small mammals.
Methods: In this 2016 cross-sectional pilot study, we sought to uniquely study wildlife for TBPs. We live-trapped small mammals, and tested their whole blood, serum and ear biopsy samples for molecular or serological evidence of Borrelia spp., Rickettsia spp., and Anaplasma spp./Ehrlichia spp.
Results: Of 64 small mammals collected, 56.0%, 39.0% and 0.0% of animals were positive by molecular assays for Borrelia spp., Rickettsia spp., and Anaplasma spp./Erhlicia spp., respectively. 41.9% were seropositive for A. phagocytophilum and 24.2% of animals were seropositive for Rickettsia rickettsii.
Conclusion: This pilot data demonstrates evidence of a number of TBPs among small mammal populations in northern Mongolia and suggests the need to further investigate what role these mammals play in human and domestic animal disease.
KEYWORDS: Anaplasma spp., Borrelia spp., Rickettsia spp., tick-borne pathogens, Mongolia, Rodentia
Background
During the last three decades, awareness of tick-borne pathogens (TBP) have increased globally. Human infection with TBPs occur when people have contact with areas cohabitated by tick vectors and disease reservoirs. Studying the ecology of TBPs and their animal reservoirs is key to our understanding of disease epidemiology. While much is known about TBPs in developed settings [1–3], major gaps of knowledge remain regarding ticks and sylvatic transmission cycles in developing countries. This holds true in Mongolia, where a number of studies documenting tick diseases of clinical relevance have confirmed the presence of TBPs [4–12], but research regarding wild animal reservoirs has been largely neglected. Further understanding of wild animal reservoirs and disease ecology of TBPs in Mongolia is particularly important, given that approximately 33% of the country’s total human population relies on herding, an activity by which individuals spend long periods of time outdoors and have increased contact with animals leading to a greater exposure to ticks.
Small mammals are known to serve as reservoirs for a variety of TBPs worldwide, including Anaplasma phagocytophilum, Borrelia spp., and Rickettsia spp., and are important to the ecology of ticks and the pathogens they harbor. Some research conducted in China has identified TBPs in small mammal species along the Mongolian border in the Xinjiang and Inner Mongolia Autonomous Regions [3,13–17]. However, few studies have assessed the prevalence of TBPs in animals, specifically small mammal species, within Mongolia, and the risk factors associated with TBP detection in animal reservoirs remains largely undescribed. In this pilot study, we sought to identify the role of small mammal species in the ecology of TBPs in Mongolia, specifically Borrelia spp., Rickettsia spp., and Anaplasma spp./Ehrlichia spp. using serological and molecular analysis.
Methods
Study sites
Between 20 June and 23 July 2016, small mammals were captured from twelve sites in three aimags (provinces) in Northern Mongolia (Figure 1). Latitude and longitude of each sampling site was collected and remote sensing data were used as previously described [18] to provide basic landscape differences among sampling areas, including normalized density vegetation index (NDVI), land surface temperature (LST), and elevation.
Figure 1.
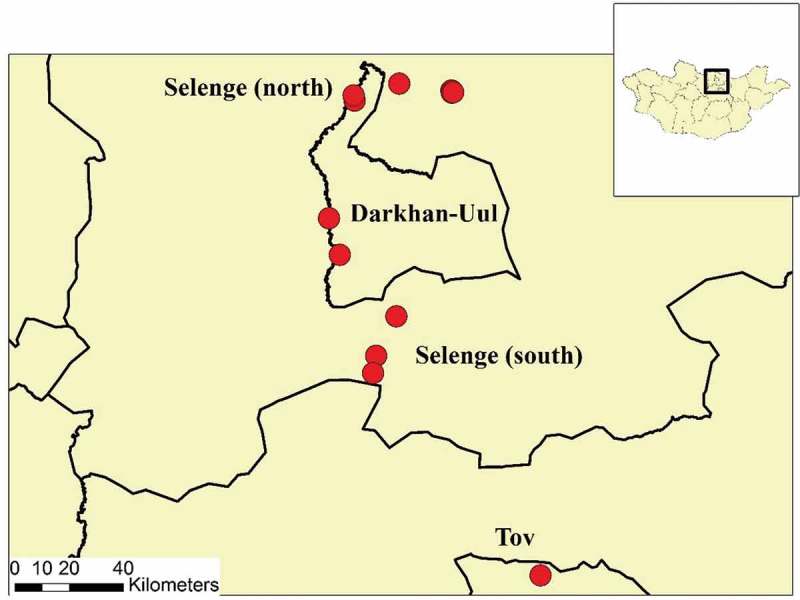
Map of animal trapping locations in northern Mongolia.
Small mammal sampling
Tomahawk traps or Sherman traps were baited with a mix of oat, grain, potato and peanut butter for 1–2 nights, for a total of 17 trap-nights. Traps were placed near small mammal burrows that had clear indications of recent small mammal activity including fresh scratched dirt and droppings in front of burrows. Traps were set between 7:00 PM and 10:00 PM and checked every 4–6 hours for the presence of small mammals.
Small mammals captured were anesthetized with ketamine (50 mg/Kg) and assessed for species and gender by a veterinarian. Samples collected from small mammals included: (1) one 2 × 2 mm ear biopsy placed in 70% EtOH, (2) one serum sample placed onto blood sampling paper (Toyo Roshi Kaisha, Ltd., Tokyo, Japan), and (3) approximately 100 uL of whole blood preserved in 20 uL 10 mM EDTA/100 uL. Whole blood samples were kept refrigerated at 4°C and ear biopsy and blood sampling paper was kept at room temperature until further analysis at the Institute of Veterinary Medicine, Ulaanbaatar, Mongolia.
PCR assays
Nucleic acid was extracted from whole blood and ear biopsy samples using the TIANamp Genomic DNA Kit (Tiangen Biotech (Beijing) Co., LTD, Beijing, China), and screened for Borrelia spp. by PCR targeting the rrs-rr1A IGS gene, as previously described (Table 1) [19]. In addition, nucleic acid extracts were screened for (1) Rickettsia spp. targeting the gltA gene (citrate synthase gene) [20] and (2) Anaplasma spp./Ehrlichia spp. targeting the 16S rRNA gene (Table 1) [21,22]. Samples were compared against known positive tick pool samples from another study that was previously sequenced and identified as Rickettsia raoultii [23], Anaplasma phagocytophilum, Ehrlichia muris, or Borrelia garinii.
Table 1.
List of primers used to conduct molecular analyses for Borrelia spp., Rickettsia spp., and Anaplasma spp./Ehrlichia spp.
| Target Gene | Primers | Sequence (5ʹ–3ʹ) | Fragment size (bp) | References |
|---|---|---|---|---|
| rrs-rr1A IGS for Borrelia spp. | BF1 | GTATGTTTAGTGAGGGGGGTG | Various | [19] |
| BR1 | GGATCATAGCTCAGGTGGTTAG | |||
| BF2 | AGGGGGGTGAAGTCGTAACAAG | |||
| BR2 | GTCTGATAAACCTGAGGTCGGA | |||
| gltA for Rickettsia spp. | CS2d | ATGACCAATGAAAATAATAAT | 381 bp | [20] |
| CSEndr | CTTATACTCTCTATGTACA | |||
| RpCS877p | GGGGACCTGCTCACGGCGG | |||
| RpCS1258n | ATTGCAAAAAGTACAGTGAACA | |||
| 16S rRNA for Anaplasma spp./Ehrlichia spp. | Ehr1 | AACGAACGCTGGCGGCAAGC | 524 bp | [21,22] |
| Ehr2 | AGTAYCGRACCAGATAGCCGC | |||
| Ehr3 | TGCATAGGAATCTACCTAGTAG | |||
| Ehr4 | CTAGGAATTCCGCTATCCTCT |
Amplified PCR products were loaded on a 2% gel for electrophoresis and stained with ethidium bromide before visualizing through an ultra violet trans-illuminator (ENDUROTM GDS, Labnet International, Edison, NJ, USA). Small mammals were determined to be actively infected with Borrelia spp., Rickettsia spp., or Anaplasma spp./Ehrlichia spp. if whole blood samples were positive by PCR. Additionally, if ear biopsy samples were positive by PCR, animals were determined positive for Borrelia spp. [24–26].
Indirect fluorescent assay
Serum samples were assessed for antibodies against Rickettsia rickettsii and Anaplasma phagocytophilum using indirect fluorescent assays (IFA). Blood sampling paper was cut into small pieces and soaked in 0.4 mL of PBS for 60 minutes at room temperature. Samples were compared against positive Rickettsia and Anaplasma serum controls (Protatek International, Inc., Minnesota, USA). Serum was diluted at 1:50 for unknown samples or 1:200 for control serum, applied to antigen slides (Protatek International, Inc., Minnesota, USA) and incubated with a wet paper towel at 37°C for 45 minutes. Slides were then washed twice in PBS on a shaker for three minutes and incubated with A/G FITC secondary conjugate (BioVision, California, USA) diluted 1:100 for R. rickettsii and 1:200 for A. phagocytophilum at 37°C for 45 minutes. Slides were washed for 3 minutes in PBS on a shaker and stained with three drops of Erichrome T-Black (Sigma-Aldrich, St. Louis, MO, USA) for 3 minutes. Slides were dried and evaluated using a fluorescent microscope. Shiny green cytoplasmic inclusion bodies, or a ‘starry night’ array, were considered positive for antibodies in serum sample against Rickettsia and Anaplasma antigen [11].
Results
A total of 64 small mammals were captured in twelve locations across three northern aimags. Vegetation differed slightly among sites with lower vegetation in Darkhan-Uul sites in comparison to Selenge and Tuv aimag (Table 2). Elevation varied the most between aimags with the highest elevation in Tuv aimag and the lowest elevation in Darkhan-Uul aimag (Table 2).
Table 2.
Landscape variables in Darkhan-Uul, Selenge and Tuv aimags. Data on mean elevation, minimum land surface temperature and maximum normalized density vegetation index was collected over a period of four years as previously described [22].
| Aimag | Mean Elevation Above Sea Level | Minimum Land Surface Temperature (°C) | Maximum Normalized Density Vegetation Index |
|---|---|---|---|
| Darkhan-Uul | 788.14 | −21.5 | 1637 |
| Selenge (north) | 832.97 | −25.6 | 1799 |
| Selenge (south) | 942.98 | −22.1 | 1827 |
| Tuv | 1343.72 | −18.7 | 1730 |
Six species of animal were captured including 21 Mongolian gerbils (Meriones unguiculatus), 19 ground squirrels (Spermophilus spp.), 17 striped dwarf hamsters (Cricetulus barabensis), 4 Siberian chipmunks (Tamius sibiricus), 2 Daurian pika (Ochotona dauurica), and one field mouse (Apodemus spp.). Of the 64 small mammals captured 62 serum samples, 61 ear biopsy samples, and 49 whole blood samples were collected.
PCR active infections
Thirty-five (56.4%) of 62 animals tested were PCR positive for Borrelia spp. in either whole blood or ear biopsy tissues of which 11/49 (22.4%) whole blood samples and 29/69 (47.5%) ear biopsy samples were positive for Borrelia spp. and five animals were positive in both whole blood and ear biopsy samples. Fourteen (74%) of 19 ground squirrels, 10 (59%) of 17 striped dwarf hamsters, 10 (50%) of 20 Mongolian gerbils, and one (25%) of four Siberian chipmunks were PCR-positive for Borrelia spp. The highest prevalence of Borrelia spp. was found in animals captured in Tuv aimag (75% among 16 animals), followed by Darkhan-Uul aimag (64% among 11 animals), and Selenge aimag (46% among 35 individual animals) (Table 3).
Table 3.
Summary of molecular and serological results by location and small mammal species.
| PCR Positive (%) |
Seropositivity (%) |
|||||
|---|---|---|---|---|---|---|
| Aimag | Species | Borrelia spp.a | Rickettsia spp. | Anaplasma spp./Ehrlichia spp. | A. phagocytophilum | R. rickettsii |
| Darkhan-Uul | Ground squirrel | 83.3% (5/6) | 0.0% (0/6) | 0.0% (0/6) | 66.7% (4/6) | 0.0% (0/6) |
| Striped dwarf hamster | 100% (1/1) | – | – | 100.0% (1/1) | 0.0% (0/1) | |
| Mongolian gerbil | 25.0% (1/4) | 75.0% (3/4) | 0.0% (0/4) | 50.0% (2/4) | 50.0% (2/4) | |
| Total Darkhan-Uul | 63.6% | 30.0% | 0.0% | 63.6% (7/11) | 18.2% (2/5) | |
| Selenge | Mongolian gerbil | 25.0% (2/8) | 100.0% (8/8) | 0.0% (0/8) | 25.0% (2/8) | 25.0% (2/8) |
| Ground squirrel | 66.7% (6/9) | 14.3% (1/7) | 0.0% (0/7) | 22.2% (2/9) | 22.2% (2/9) | |
| Striped dwarf hamster | 50.0% (7/14) | 9.1% (1/11) | 0.0% (0/11) | 50.0% (7/14) | 28.6% (4/14) | |
| Siberian chipmunk | 25.0% (1/4) | 0.0% (0/3) | 0.0% (0/3) | 50.0% (2/4) | 25.0% (1/4) | |
| Total Selenge | 45.7% | 34.5% | 0.0% | 37.1% (13/35) | 25.7% (9/35) | |
| Tuv | Ground squirrel | 75.0% (3/4) | 0.0% (0/4) | 0.0% (0/4) | 75.0% (3/4) | 50.0% (2/4) |
| Daurian pika | 0.0% (0/1) | – | – | 0.0% (0/1) | 0.0% (0/1) | |
| Field mouse | 0.0% (0/1) | – | – | 0.0% (0/1) | 0.0% (0/1) | |
| Mongolian gerbil | 87.5% (7/8) | 100.0% (6/6) | 0.0% (0/6) | 12.5% (1/8) | 25.0% (2/8) | |
| Striped dwarf hamster | 100.0% (2/2) | – | – | 100.0% (2/2) | 0.0% (0/2) | |
| Total Tuv | 75.0% | 60.0% | 0.0% | 37.5% (6/16) | 25.0% (4/16) | |
| Grand Total | 56.4% (35/62) | 38.8% (19/49) | 0.0% (0/49) | 41.9% (26/62) | 24.2% (15/62) | |
aIndicates molecular detection in whole blood or ear biopsy samples.
Nineteen (39%) of 49 whole blood samples were PCR-positive for Rickettsia spp., of these 17/18 (94.4%) were from Mongolian gerbils, 1/11 (9.1%) from striped dwarf hamsters, and 1/17 (5.9%) from ground squirrels. Animals captured in Tuv aimag had the highest prevalence of Rickettsia spp. (60% among 10 animals), followed by Selenge aimag (34% among 29 animals) and Darkhan-Uul aimag (30% among 10 animals) (Table 3). No samples tested positive by PCR for Anaplasma spp./Ehrlichia spp.
Seropositivity of tick-borne pathogens in small mammals
Evidence of previous infection of Rickettsia spp. was detected in serum of 15 (24.2%) of 62 animals consisting of 6 (30%) of 20 Mongolian gerbils, 1 (25%) of 4 Siberian chipmunks, 4 (23.5%) of 17 striped dwarf hamsters, and 4 (21.0%) of 19 ground squirrels. Animals captured in Selenge aimag had the highest prevalence of previous infection with Rickettsia spp. (26.0%, 9/35 animals), followed by Tuv aimag (25.0%, 4/16 animals) and Darkhan-Uul aimag (18.0%, 2/11 animals) (Table 3).
Evidence of previous infection with Anaplasma spp. was present in serum of 26 (41.9%) of 62 serum samples collected (Table 3). Of these, 10 (58.8%) of 17 striped dwarf hamsters, 2 (50%) of 4 Siberian chipmunks, 9 (47.4%) of 19 ground squirrels, and 5 (25%) of 20 Mongolian gerbils had antibodies present for Anaplasma spp. Seroprevelance of Anaplasma spp. was highest in small mammals captured in Darkhan-Uul aimag (64.0%, 7/11 animals), followed by Tuv aimag (38.0%, 6/16 animals) and Selenge aimag (37.0%, 13/35 animals) (Table 3).
Discussion
This report is part of a limited body of research describing the prevalence of tick-borne pathogens among small mammal reservoirs in Mongolia. While speciation of pathogens was not possible in this pilot study, PCR results suggest that there is a high prevalence of Borrelia spp. (56.4%) and Rickettsia spp. (38.8%) in a number of small mammal species captured in this study. There was also high geographic distribution of infection of Borrelia spp. (46.0–75%) and Rickettsia spp. (30–60%). There was no molecular evidence of Anaplasma spp./Ehrlichia spp. in any host species, however serum samples collected had a high antibody response to A. phagocytophilum (41.9%).
Small mammals as competent reservoirs of tick-borne pathogens
Prior studies identified a high prevalence of Borrelia spp. in I. pursulcatus ticks in Selenge aimag further providing evidence that rodents likely harbor Borrelia spp. in northern Mongolia [9,10]. While Dermacentor spp. was the only tick species identified on animals captured during this study, I. persculcatus ticks were present in these sampled areas suggesting that Borrelia spp. were also likely present in animal reservoirs captured [3]. The most common strains of Borrelia spp. circulating in Mongolia are B. afzelii, B. bavariensis and B. garinii [9,10], all of which are reservoired in rodents and are commonly found to transmit to humans. B. garinii in particular has been identifed in hamster [13,27] and sibirian chipmunk species [26] in neighborhing China. While ground squirrels and Mongolian gerbils have not been identified as hosts of Borrelia in Mongolia’s neighborhing countries, research out of the United States has found California gray squirrels as competant reservoirs for Borrelia spp. [28] and Mongolian gerbils have historically been used in experimental studies as reservoirs for Borrelia spp. [29,30] suggesting that they too could be competant reservoirs.
Prior studies found a large variation of prevalence of infection of Rickettsia spp. in ticks ranging from 12.5% to 97.0% in northern Mongolia [5,14,31]. Rickettsia raoultii is the dominant SFGR species circulating in D. nuttalii ticks in northern Mongolia with prevalence ranging between 66 and 97.0% [5,12] suggesting that Rickettsia raoultii could be circulating in small mammals captured in this study. In our study, Mongolian gerbils had a very high prevalence of Rickettsia spp. in comparison to other small mammal species. While we could not find supporting evidence for spotted fever group rickettsiosis (SFGR) circulating in Mongolian gerbils in surrounding regions, they have been known to reservoir SFGR in other parts of the world [32]. Other rodents have also been found to reservoir SFGR in China, suggesting that our rodent population could be reservoiring SFGR [16]. However, due to financial constraints and time we were not able to sequence samples and therefore it is likely that we are detecting other rickettsial species such as scrub typhus, Orientia tsutsugamushi, which has been identified in Inner Mongolia and is known to circulate in small mammals [2]. Therefore, we cannot definitively say that SFGR is circulating in the population but suggest that further research is warranted to assess this question further as SFGR is highly prevalent in ticks in this area.
While we did not identify any molecular evidence of Anaplasma spp./Ehrlichia spp. in reservoir hosts we found a high response to antibody response to A. phagocytophilum. Previous studies in the United Kingdom found that prevalence rates in rodents was highly seasonal and could be linked to peak nymphal or adult tick seasons [33]. Most of the ticks found on the rodents captured in this study were larvae which could be why we don’t see any active infections in captured rodents but did see antibody responses to A. phagocytophilum. Anaplasma spp. has also been shown to have variable durations of infection with some rodent species having longer persistence of infection than others [33]. This could in part be due to their immunity to infection or other attributes such as size of rodent. For example, it is thought that older animals may have a higher immunity to infection in comparison to juveniles [33,34]. Alternatively, larger rodents might be more susceptible to infection based on the mere fact that they can sustain a larger population of ticks and hence are more likely to become infected. Previous reports regarding the prevalence of Anaplasma spp. in Mongolia have also found similarly low prevalence in ticks suggesting that Anaplasma spp. may not be highly prevalent in small mammals in this area or it is highly seasonal [4,9,35]. In regards to reservoir competence, striped dwarf hamsters, Siberian chipmunks and ground squirrels had a high seroprevalence of A. phagocytophilum, similar studies in China found that gerbils and Siberian chipmunks were adequate hosts for A. phagocytophilum [13,16]. In the United States, Spermophilus spp. are similarly competent reservoir hosts for A. phagocytophilum [36] suggesting that they too could sustain transmission of Anaplasma spp. in our study area.
Implications of tick-borne pathogens on humans and domestic animals
Few studies have assessed TBP prevalence among the Mongolian population and its domestic livestock. The few studies which have assessed this found Borrelia spp. in the general population with incidences as high as 7.8 cases per 100,000 persons per year in Selenge aimag [10] and seroprevalences of A. phagocytophilum ranging between 2.3 and 37.3% throughout the country [6,23]. Relatively less is known regarding the prevalence of SFGR in humans, however case studies have identified infections of Rickettsia siberica in international travelers visiting Mongolia and a recent study found high seroprevalences of SFGR among humans especially in the northern provinces of Mongolia [7,8,23].
As a large proportion of the population rely on herding, the impact of TBPs on domestic livestock is also of interest given that domestic animals can contract TBPs [1,13,24,37] and can serve as a vector for the transfer of infected ticks to humans. Recent studies of domestic animals in Mongolia identified antibody responses to B. burgdorferi, SFGR, and Anaplasma spp. in various domestic livestock in northern Mongolia [11,23]. These findings are similar to the seroprevalences of R. rickettsii (24.2%) and A. phagocytophilum (41.9%) we found in our small mammal population suggesting that these pathogens are likely shared between wildlife reservoirs and domestic animals in this region.
Apart from the seroprevalence of A. phagocytophilum, TBP prevalence was highest in small mammals captured in Tuv aimag. Tuv aimag surrounds the capitol of Mongolia, Ulaanbaatar, and therefore might have a higher distribution of domestic animals moving in and out of this region. As domestic animals play an important role in the tick life cycle this location may be prime tick habitat potentially leading to a higher prevalence of TBPs. Further studies need to address areas such as this where there is a large influx of humans and animals moving through this landscape.
Conclusion
This pilot study identified the need to further explore Borrelia spp. and Rickettsia spp. circulating in small mammal reservoirs in northern Mongolia. Additional studies are needed to further assess specific species of TBPs present in small mammal reservoirs in Mongolia. Specifically, studies with larger sample sizes are needed, particularly in areas where small mammal reservoirs, domestic animals, humans and ticks overlap. Such studies are necessary to further understand the complexity of tick-borne pathogens in Mongolia.
Funding Statement
Funding for this work was provided by the Duke Global Health Institute, Duke University.
Acknowledgments
This study was made possible due to the assistance and support provided by the Mongolian Institute of Veterinary Medicine, Duke One Health Laboratory, and Duke Global Health Institute.
Authors’ contributions
Study design: LP, TM, MVF, BA, BG and GCG; sample collection: LP, TM and LC; Lab analysis: LP, TM and LS; manuscript preparation: LP. All authors have read and approved the final version of this manuscript.
Ethics approval and consent to participate
Trapping and handling procedures were approved by the Duke University Institutional Animal Care and Use Committee (#A086-16-04).
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1]. Steere AC, Coburn J, Glickstein L.. The emergence of Lyme disease. J Clin Invest. 2004. April;113(8):1093–7. PubMed PMID: 15085185; PubMed Central PMCID: PMCPMC385417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Comstedt P, Jakobsson T, Bergstrom S. Global ecology and epidemiology of Borrelia garinii spirochetes. Infect Ecol Epidemiol. 2011;1 DOI: 10.3402/iee.v1i0.9545 PubMed PMID: 22957111; PubMed Central PMCID: PMCPMC3426327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Swanson SJ, Neitzel D, Reed KD, et al. Coinfections acquired from ixodes ticks. Clin Microbiol Rev. 2006. October;19(4):708–727. PubMed PMID: 17041141; PubMed Central PMCID: PMCPMC1592693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Javkhlan G, Enkhtaivan B, Baigal B, et al. Natural Anaplasma phagocytophilum infection in ticks from a forest area of Selenge province, Mongolia. Western Pac Surveill Response J. 2014;5(1):21–24. PubMed PMID: 24734213; PubMed Central PMCID: PMCPMC3984964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Speck S, Derschum H, Damdindorj T, et al. Rickettsia raoultii, the predominant Rickettsia found in Mongolian Dermacentor nuttalli. Ticks Tick Borne Dis. 2012. September;3(4):227–231. PubMed PMID: 22784401; Eng. [DOI] [PubMed] [Google Scholar]
- [6]. Walder G, Lkhamsuren E, Shagdar A, et al. Serological evidence for tick-borne encephalitis, borreliosis, and human granulocytic anaplasmosis in Mongolia. Int J Med Microbiol. 2006. May;296(Suppl 40):69–75. PubMed PMID: 16524782. [DOI] [PubMed] [Google Scholar]
- [7]. Lankester T, Davey G. A lump on the head from Mongolia. Lancet. 1997. March 1;349(9052):656 PubMed PMID: 9057768; Eng. [DOI] [PubMed] [Google Scholar]
- [8]. Lewin MR, Bouyer DH, Walker DH, et al. Rickettsia sibirica infection in members of scientific expeditions to northern Asia. Lancet. 2003. October 11;362(9391):1201–1202. PubMed PMID: 14568744; Eng. [DOI] [PubMed] [Google Scholar]
- [9]. Masuzawa T, Masuda S, Fukui T, et al. PCR detection of Anaplasma phagocytophilum and Borrelia burgdorferi in Ixodes persulcatus ticks in Mongolia. Jpn J Infect Dis. 2014;67:47–49. [DOI] [PubMed] [Google Scholar]
- [10]. Scholz HC, Margos G, Derschum H, et al. High prevalence of genetically diverse Borrelia bavariensis-like strains in Ixodes persulcatus from Selenge Aimag, Mongolia. Ticks Tick Borne Dis. 2013. February;4(1–2):89–92. PubMed PMID: 23084366. [DOI] [PubMed] [Google Scholar]
- [11]. Papageorgiou S, Battsetseg G, Kass Philip H, et al. Detection and epidemiology of tick-borne pathogens in free-ranging livestock in Mongolia. J Clin Exp Pathol. 2013;01(S3). doi: 10.4172/2161-0681.s3-006. [DOI] [Google Scholar]
- [12]. Boldbaatar B, Jiang R-R, von Fricken ME, et al. Distribution and molecular characteristics of rickettsiae found in ticks across central Mongolia. Parasit Vectors. 2017. February 2;10(1):61 PubMed PMID: 28153052; Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Fang L-Q, Liu K, Li X-L, et al. Emerging tick-borne infections in mainland China: an increasing public health threat. Lancet Infect Dis. 2015;15(12):1467–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Liu L, Chen Q, Yang Y, et al. Investigations on Rickettsia in ticks at the Sino-Russian and Sino-Mongolian borders, China. Vector Borne Zoonotic Dis. 2015. December;15(12):785–789. PubMed PMID: 26684526. [DOI] [PubMed] [Google Scholar]
- [15]. Chu C-Y, Jiang B-G, Liu W, et al. Presence of pathogenic Borrelia burgdorferi sensu lato in ticks and rodents in Zhejiang, south-east China. J Med Microbiol. 2008. August;57(Pt 8):980–985. PubMed PMID: 18628499. [DOI] [PubMed] [Google Scholar]
- [16]. Zhan L, Cao WC, Chu CY, et al. Tick-borne agents in rodents, China, 2004–2006. Emerg Infect Dis. 2009. December;15(12):1904–1908. PubMed PMID: 19961668; PubMed Central PMCID: PMCPmc3044509. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Zhang F, Gong Z, Zhang J, et al. Prevalence of Borrelia burgdorferi sensu lato in rodents from Gansu, northwestern China. BMC Microbiol. 2010. May 28;10:157 PubMed PMID: 20509909; PubMed Central PMCID: PMCPmc2889951. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Hay SI, Tatem AJ, Graham AJ, et al. Global environmental data for mapping infectious disease distribution. Adv Parasitol. 2006;62:37–77. PubMed PMID: PMC3154638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Bunikis J, Garpmo U, Tsao J, et al. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology. 2004;150(6):1741–1755. [DOI] [PubMed] [Google Scholar]
- [20]. Mediannikov OY, Sidelnikov Y, Ivanov L, et al. Acute tick-borne Rickettsiosis caused by Rickettsia heilongjiangensis in Russian Far East. Emerg Infect Dis. 2004;10(5):810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Rar VA, Livanovab NN, Panovb VV, et al. Prevalence of Anaplasma and Ehrlichia species in Ixodes persulcatus ticks and small mammals from different regions of the Asian part of Russia. Int J Med Microbiol. 2008;298:222–230. [Google Scholar]
- [22]. Rar VA, Livanova NN, Panov VV, et al. Genetic diversityof Anaplasma and Ehrlichia in the Asian part of Russia. Ticks Tick Borne Dis. 2010;1(1):57–65. [DOI] [PubMed] [Google Scholar]
- [23]. von Fricken ME, Lkhagvatseren S, Boldbaatar B, et al. Estimated seroprevalence of Anaplasma spp. and spotted fever group Rickettsia exposure among herders and livestock in Mongolia. Acta Trop. 2018. January;177:179–185. PubMed PMID: 29054570; PubMed Central PMCID: PMC5671362. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Barbour AG, Bunikis J, Travinsky B, et al. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009. December;81(6):1120–1131. PubMed PMID: 19996447; PubMed Central PMCID: PMCPMC2841027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Gassner F, Takken W, Plas CL, et al. Rodent species as natural reservoirs of Borrelia burgdorferi sensu lato in different habitats of Ixodes ricinus in The Netherlands. Ticks Tick Borne Dis. 2013. September;4(5):452–458. PubMed PMID: 23891104; Eng. [DOI] [PubMed] [Google Scholar]
- [26]. Marsot M, Sigaud M, Chapuis JL, et al. Introduced Siberian chipmunks (Tamias sibiricus barberi) harbor more-diverse Borrelia burgdorferi sensu lato genospecies than native bank voles (Myodes glareolus). Appl Environ Microbiol. 2011. August 15;77(16):5716–5721. PubMed PMID: 21705536; PubMed Central PMCID: PMCPMC3165248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Takada N, Masuzawa T, Ishiguro F, et al. Lyme disease Borrelia spp. in ticks and rodents from northwestern China. Appl Environ Microbiol. 2001. November;67(11):5161–5165. PubMed PMID: 11679340; PubMed Central PMCID: PMCPMC93285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Lane RS, Mun J, Eisen RJ, et al. Western gray squirrel (Rodentia: Sciuridae): a primary reservoir host of Borrelia burgdorferi in Californian oak woodlands? J Med Entomol. 2005. May;42(3):388–396. PubMed PMID: 15962792; Eng. [DOI] [PubMed] [Google Scholar]
- [29]. Gray JS, Schönberg A, Postic D, et al. First isolation and characterisation of Borrelia garinii, agent of Lyme borreliosis, from Irish ticks. Ir J Med Sci. 1996. Jan-Mar;165(1):24–26. PubMed PMID: 8867493; Eng. [DOI] [PubMed] [Google Scholar]
- [30]. Matuschka FR, Schinkel TW, Klug B, et al. Relative incompetence of european rabbits for Lyme disease spirochaetes. Parasitology. 2000. September;121(Pt 3):297–302. PubMed PMID: 11085249; Eng. [DOI] [PubMed] [Google Scholar]
- [31]. Narantsatsral S, Myagmarsuren P, Davaasuren P, et al. Molecular biological detection of emerging tick-borne zoonotic pathogens in ixodid tick species. Mongolian J Agric Sci. 2014;13(2):3–7. [Google Scholar]
- [32]. Shoukry A, Merdan AI, el-Kady GA. The role of rodents as reservoirs for some diseases in new reclaimed areas in north Sinai. J Egypt Soc Parasitol. 1991. August;21(2):513–519. PubMed PMID: 1908502; Eng. [PubMed] [Google Scholar]
- [33]. Bown KJ, Begon M, Bennett M, et al. Seasonal dynamics of Anaplasma phagocytophila in a rodent-tick (Ixodes trianguliceps) system, United Kingdom. Emerg Infect Dis. 2003. January;9(1):63–70. PubMed PMID: 12533283; PubMed Central PMCID: PMCPmc2873734. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Chastagner A, Moinet M, Perez G, et al. Prevalence of Anaplasma phagocytophilum in small rodents in France. Ticks Tick Borne Dis. 2016. July;7(5):988–991. PubMed PMID: 27270190. [DOI] [PubMed] [Google Scholar]
- [35]. Karnath C, Obiegala A, Speck S, et al. Detection of Babesia venatorum, Anaplasma phagocytophilum and Candidatus Neoehrlichia mikurensis in Ixodes persulcatus ticks from Mongolia. Ticks Tick Borne Dis. 2016. March;7(2):357–360. PubMed PMID: 26739031. [DOI] [PubMed] [Google Scholar]
- [36]. Adjemian JZ, Adjemian MK, Foley P, et al. Evidence of multiple zoonotic agents in a wild rodent community in the eastern Sierra Nevada. J Wildl Dis. 2008. July;44(3):737–742. PubMed PMID: 18689664. [DOI] [PubMed] [Google Scholar]
- [37]. Littman MP, Goldstein RE, Labato MA, et al. ACVIM small animal consensus statement on Lyme disease in dogs: diagnosis, treatment, and prevention. J Veter Intern Med. 2006. Mar-Apr;20(2):422–434. PubMed PMID: 16594606; Eng. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


