Figure 2. Accumulating vs, abrupt switches in integrin avidity states in rolling leukocytes.
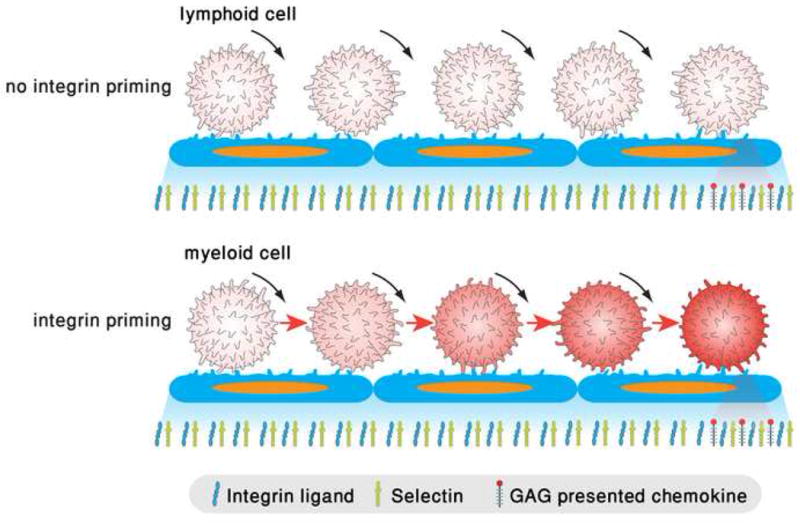
Upper panel: A lymphocytes rolls via PSGL-1 or other endothelial selectin ligands (not shown) but fails to undergo integrin activation by successive rolling engagements due to insufficient triggering of kinases, PLCs and secondary messengers. When lymphocytes encounter high density of arrest chemokines juxtaposed to integrin ligands, their integrins are locally and instantaneously activated by Gαiβγ signals as delineated in figure 1. Lower panel: a rolling neutrophil and possibly other myeloid leukocytes can integrate weak integrin activation signals through engagements of E-selectin ligands including PSGL-1, which trigger phosphorylation and activation of spleen tyrosine kinase Syk [8,58]. Within seconds, LFA-1 and possibly other integrins are stabilized in an extended intermediate affinity state on the entire plasma membrane. These integrins can form reversible adhesive bonds with endothelial ICAM-1 sufficient to slow down selectin mediated rolling. Like the lymphocyte, when the rolling neutrophil encounters high density of arrest chemokines, its integrins can undergo robust bidirectional activation via Gi signals. Post arrest, ligand-driven integrin microclustering can immediately follow to further stabilize the integrin-mediated contact. Co-ligation of multiple selectin ligands and integrins trigger both Src and Syk kinases to further activate integrin avidity and adhesion strengthening [59].
