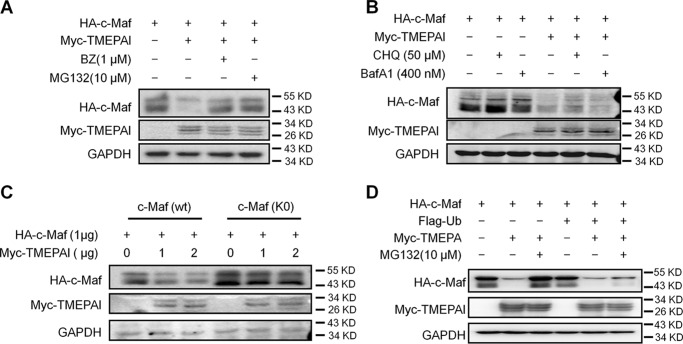
Figure 2.
TMEPAI induces c-Maf degradation via the proteasomal but not the lysosomal pathway. A, c-Maf and TMEPAI plasmids were co-transfected into HEK293T cells, followed by the treatment of proteasomal inhibitors BZ or MG132. The cell lysates were subjected to immunoblotting assay. B, c-Maf and TMEPAI plasmids were co-transfected into HEK293T cells, followed by the treatment of lysosomal inhibitors chloroquine (CHQ) or bafilomycin A1 (BafA1). The cell lysates were subjected to immunoblotting assay. C, TMEPAI was co-transfected into HEK293T cells with the WT or all-lysine mutant c-Maf (K0) plasmids for 24 h followed by immunoblotting assay. The relative expression of c-Maf protein against GAPDH is shown at the bottom. D, a c-Maf plasmid was co-transfected into HEK293T cells with a TMEPAI plasmid with or without ubiquitin (Ub) plasmid transfection. Twenty-four hours later, the cells were treated with MG132 for 8 h before being applied to an immunoblotting assay for indicated protein expression.