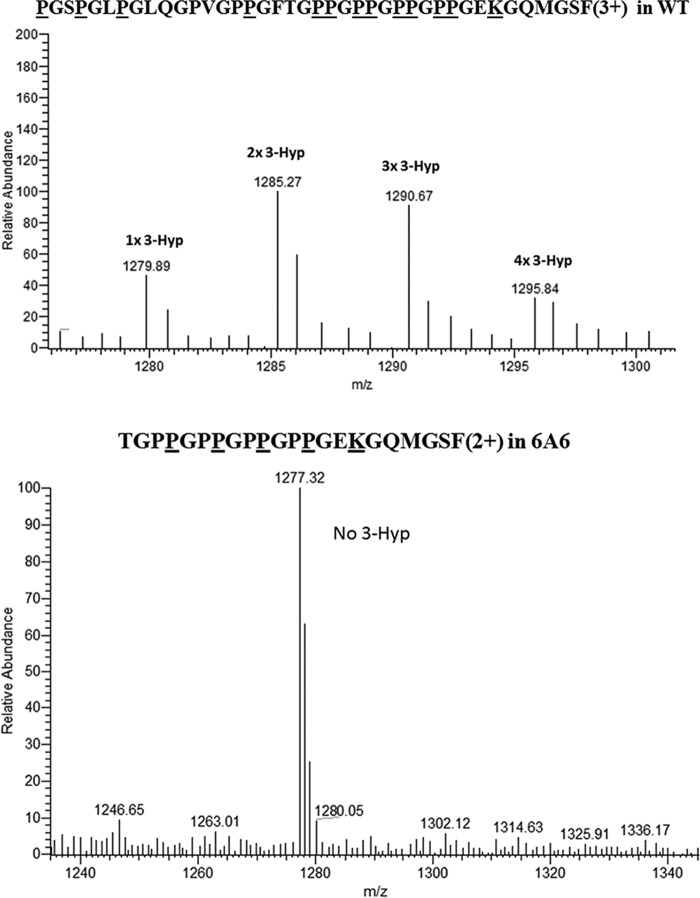
Figure 3.
Extent of hydroxylation in WT and P3H2 KO type IV collagen GPPGPPGPPGPP peptide. Top panel, averaged MS scans from m/z 1275 to 1300 in chromatograms where CNBr + tryptic type IV collagen α1 hydroxylated peptide from WT cells elute. Underlined residues in the amino acid sequence show predicted 3-and 4-hydroxproline modification sites, as well as a modified glucosylgalacotosyl hydroxylysine and oxidized methionine. Bottom panel, averaged MS scans from m/z 1250 to 1300 where chymotryptic type IV collagen α1 peptide from P3H2 KO cells elute. Underlined residues in the amino acid sequence show predicted 4-hydroxyproline modification sites and modified glucosylgalacotosyl hydroxylysine. Residues Pro204, Pro207, Pro210, and Pro213 in the WT peptide were at least partially hydroxylated, as the indicated masses corresponding to a mixture of peptides containing 2–5 extra hydroxylation sites, whereas these putative 3-hydroxproline sites were not observed in P3H2 KO peptides.