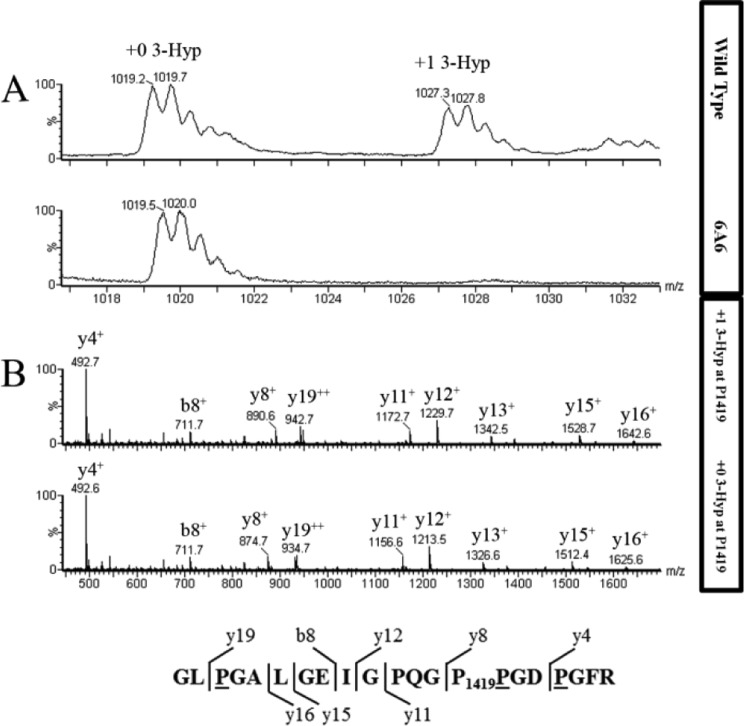
Figure 4.
Extent of hydroxylation in type IV collagen α-2 residue P1419 from WT and P3H2 KO cells. A, averaged MS scans from m/z 1016 to 1034 in chromatograms where tryptic type IV collagen α2 peptide from WT (top panel) and P3H2 KO (bottom panel) cells elute. Amino acid sequence is shown with observed b and y ions. B, fragment spectrum for this peptide from m/z 450 to 1700 for WT (top panel) and P3H2 KO (bottom panel) cells. The b and y ions and their m/z values are indicated for each labeled peak. Underlined residues in the amino acid sequence at the bottom are predicted to be hydroxylated, and Pro1419 is predicted to be 3-hydroxylated, based on the +16 unit mass shifts of the y8 fragment ion and larger y ions in the peptide from WT cells. As expected, peptides from both WT and KO cells contain three 4-Hyp residues.