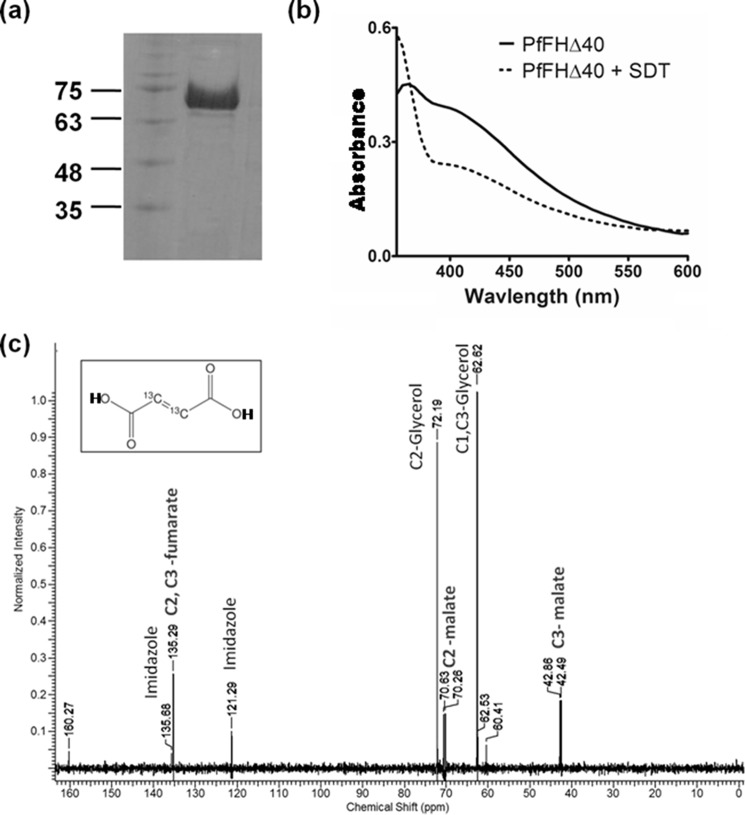
Figure 3.
Purification and activity of PfFHΔ40. a, 1st lane, protein molecular mass markers (numbers indicated are in kDa); 2nd lane, Ni-NTA-purified PfFHΔ40. b, UV-visible absorption spectrum of purified and reconstituted PfFH shows characteristic peaks at 360 and at 405 nm that indicate the presence of a 4Fe-4S cluster. The spectrum of the protein with oxidized iron–sulfur cluster is shown as a solid line and that with reduced iron–sulfur cluster upon addition of 1 mm sodium dithionite (SDT) is shown as a dashed line. c, validation of malic acid formation by 13C NMR. The NMR spectrum of assay mixture consisting of 50 μm 2,3-[13C]fumaric acid in 100 mm potassium phosphate, pH 7.4, incubated with 100 μg of purified PfFHΔ40 enzyme, shows the presence of peaks corresponding to [13C]malic acid. Unreacted [13C]fumaric acid is also present. The inset shows the chemical structure of [13C]fumaric acid. The spectrum is an average of 3000 scans acquired using Bruker 400-MHz NMR spectrometer. The peaks corresponding to imidazole and glycerol are from the protein solution.