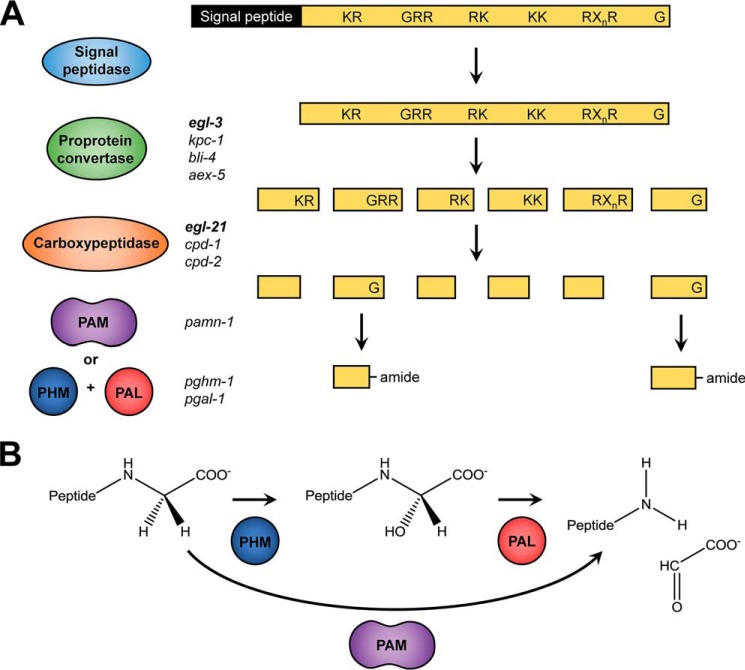
Figure 1.
Neuropeptide-processing pathway. Neuropeptides are synthesized as large preproproteins that require post-translational processing, and as exemplified in A, the signal peptide is cleaved upon entry into the secretory pathway by a signal peptidase. Subsequently, a proprotein convertase (mainly egl-3, but also kpc-1, bli-4, and aex-5 in C. elegans) cleaves the remaining part of the precursor protein at specific motifs containing basic amino acids (KR, RR, RK, KK, or RXnR with n = 2, 4, 6, or 8). These residues are then removed by a carboxypeptidase (EGL-21, CPD-1 and CPD-2 in C. elegans) to yield the cleaved peptide. Finally, the carboxyl-terminal glycine residue, if present, is transformed into an amide. B, carboxyl-terminal amidation involves two steps: hydroxylation of the glycine α-carbon by a PHM, followed by a cleavage reaction performed by a PAL. This will generate a glyoxylate molecule and the α-amidated peptide. In vertebrates, these two enzymatic activities are contained in one bifunctional enzyme, PAM.