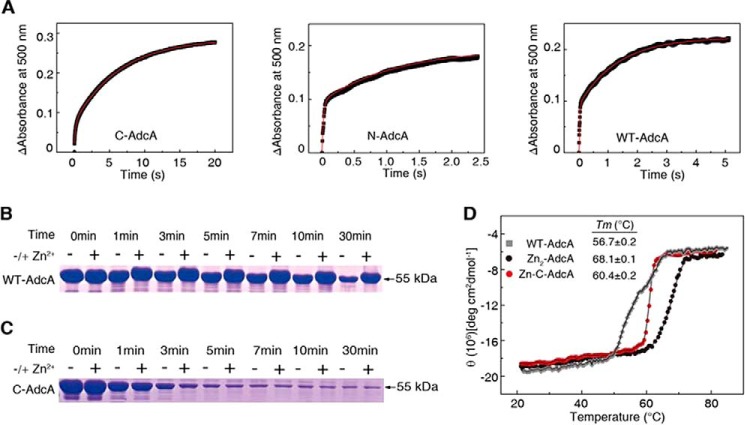
Figure 4.
Biochemical, thermodynamic, and kinetic characterization to verify protein stability. A, kinetics of AdcA binding to zinc. Time-dependent reactions were created using 10 μm apo-form proteins mixed with 40 μm Zn(PAR)2. B, proteinase K sensitivity of WT AdcA. Proteins (15 μg) were subjected to proteinase K (30 μg/liter) for 0, 1, 3, 5, 7, 10, and 30 min. C, proteinase K sensitivity of C-AdcA. D, thermal stabilities of WT AdcA, Zn2-AdcA, and Zn-C-AdcA. Thermal unfolding transitions were monitored using far-UV CD spectra at 223 nm. deg, degrees.