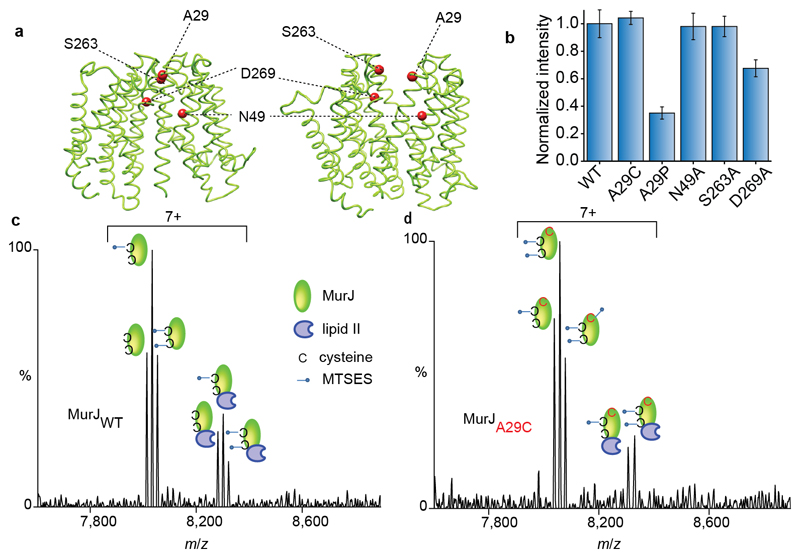
Figure 5. Structures of MurJ with residues investigated through mutation, their effects on lipid II binding and spectra recorded for wild type and A29C after treating with MTSES in the presence of lipid II.
a Structures of MurJ in inward and outward facing forms were generated using I-TASSER and the structure PDB 5T77 and outward parameters of MurJTA (17). Mutated residues are shown as red spheres. b The effect of mutations on lipid II binding normalised to the wild type. N49A and S263A have no effect whereas A29P and D269A both have attenuated lipid II binding. Error bars represent standard deviations (n=3). c Mass spectrum recorded for MTSES treated wild-type MurJ. Both native cysteine residues are modified (second and third peak in the left set) and some unmodified protein remains leading to a triplet. All forms of the protein retain competency to bind lipid II (three peaks in the right hand set). d Mass spectrum for A29C MurJ variant with all three cysteine residues modified by MTSES (first, second and third peaks in the left set). In this case only two of the three modified sites are capable of binding to lipid II (first and second peaks in the right set) implying that the A29C mutant is unable to bind lipid II. All experiments were performed three times and spectra are representative.