Figure 2.
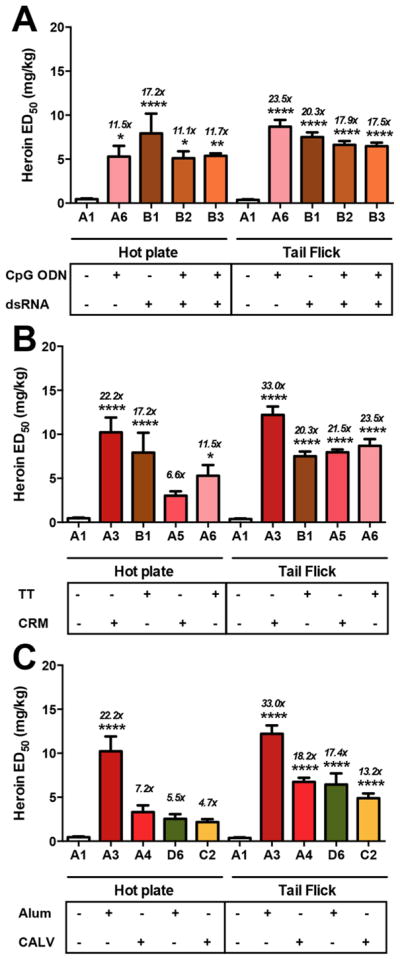
Effects of adjuvants and carrier proteins on heroin vaccine efficacy in antinociception assays. Differences in formulation are shown below the x-axis in each panel. A1 is a vehicle control. Panel A shows the effects of RNA versus DNA. All vaccines contain 50 μg Her-TT and 1 mg of alum. Only B3 used trehalose as a cryoprotectant for lyophilization treatment; all other vaccines contained glycerol. Panel B shows the effect of carrier protein. All vaccines contain 50 μg of immunoconjugate, 1 mg of alum and glycerol. A3 and B1 contain 50 μg of dsRNA, and A5 and A6 contain 50 μg of CpG. Panel C displays the effect of alum versus CALV as delivery vehicles. All vaccines contain 50 μg dsRNA. Groups A3 and A4 contain 50 μg of Her-CRM, and groups D6 and C2 contain 50 μg of Her-TT. Italicized numbers above the bars represent the ED50 ratio versus nonvaccinated control animals from control A1. A one-way ANOVA was performed for each antinociception assay, followed by a Dunnett’s post hoc comparison test, respectively. *P < 0.05, **P < 0.01, ****P < 0.0001 versus control A1.
