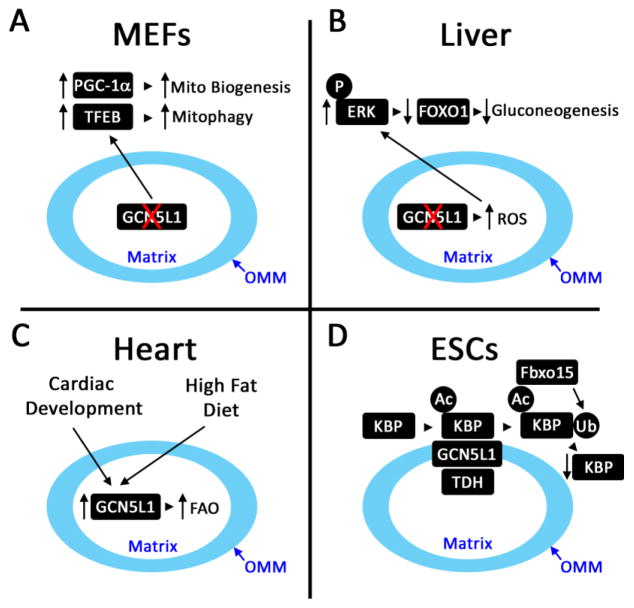
Figure 1. Schematic of Proposed Functions of Mitochondrial GCN5L1.
A. In mouse embryonic fibroblasts (MEFs) and transformed cell lines, the genetic depletion of GCN5L1 reduced mitochondrial protein acetylation in parallel with induction of mitochondrial biogenesis (PGC-1α) and lysosomal biogenesis (TFEB) regulatory programs. This effect is postulated to result from retrograde signaling from the michondria to nucleus, although the signaling pathway have not been defined. B. The role of mitochondrial signaling from the disruption of GCN5L1 levels was further expanded upon in the regulation of transcriptional regulation of gluconeogenesis. Here, in primary hepatocytes, the genetic depletion of GCN5L1 increased mitochondrial ROS-driven ERK signaling which promoted FoxO1 degradation and the subsequent downregulation in hepatic gluconeogenesis. C. Physiologic studies showed that during heart development or in response to high fat feedings cardiac GCN5L1 levels were increased and this was linked to increased fatty acid oxidation enzyme acetylation and activity. D. On the outer mitochondrial membrane (OMM) in embryonic stem cells (ESCs) GCN5L1 functions in concert with the acetyl-CoA generating enzyme L-threonine dehydrogenase (TDH) to acetylate a kinesin binding protein (KBP). This modification in turn promoted the proteasomal degradation of KBP via the ubiquitin ligase Fbxo15. In the absence of GCN5L1 the levels of KBP are maintained which drove mitochondrial biogenesis.