Figure 2.
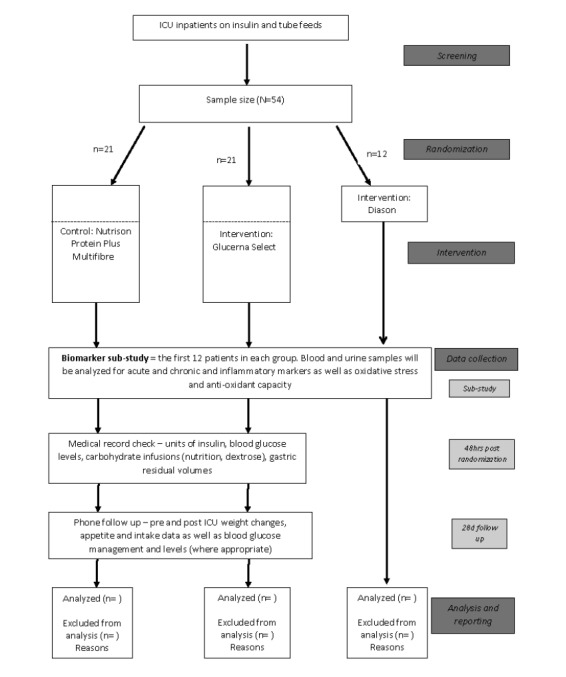
Study flow diagram of recruitment, randomization and study conduct. Once consented patients will be randomized on a 1:1:1 ratio until there are 12 patients who have complete blood and urine sample sets from each group to form the biomarker sub-study. Thereafter the study will proceed with two arms as indicated. Finalized patient numbers have not been provided as this is a feasibility study.
