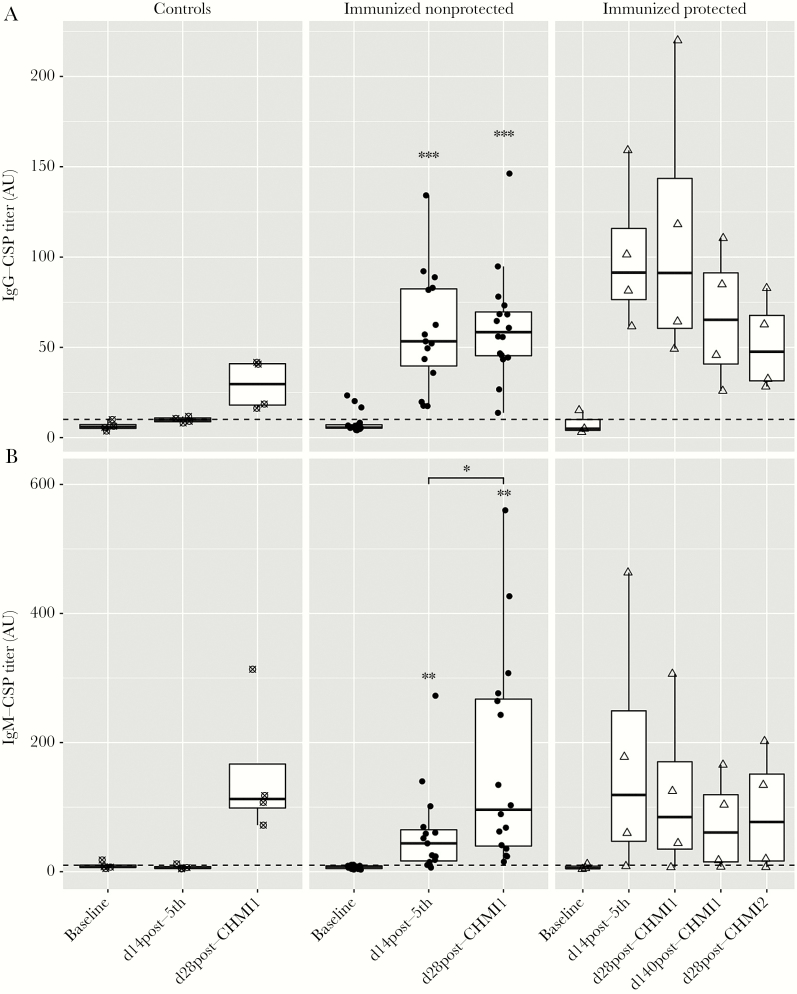
Figure 2.
Induction of IgG (A) and IgM antibody (B) responses to full-length P. falciparum circumsporozoite protein (CSP) from malaria-preexposed volunteers immunized with 5 × 2.7 × 105P. falciparum sporozoites (PfSPZ) Vaccine, quantified by enzyme-linked immunosorbent assay (ELISA) at the time points: baseline, day 14 post-fifth immunization, day 28 post-controlled human malaria infection 1 (CHMI1), day 140 post-CHMI1, and day 28 post-CHMI2. Antibody titers are specified as arbitrary units (AU) with a positivity cutoff shown by a dashed line. Each data point represents the mean of the duplicate ELISA titers for 1 volunteer. Asterisks indicate statistically significant difference of the mean antibody titer compared to the mean titer measured at the pre-immunization time point as determined by paired t test. Bars with asterisks indicate statistically significant difference of the mean antibody titer between visits determined by paired t test. *P ≤ .05, **P ≤ .01, ***P ≤ .001. The small sample size of control and protected subjects did not allow for statistical testing when grouped by protection status. For whole-group analyses, see Supplementary Figure 3. Control volunteers are shown as crossed circles (n = 4) in the left panel, immunized nonprotected volunteers as solid circles (n = 16) in the middle panel, and immunized protected (n = 4) as empty triangles in the right panel. Serum samples of 2 volunteers were not available, one at baseline (protected subject) and one at visit of day 14 post-fifth (nonprotected subject). The 2 volunteers were not considered for statistical analysis.