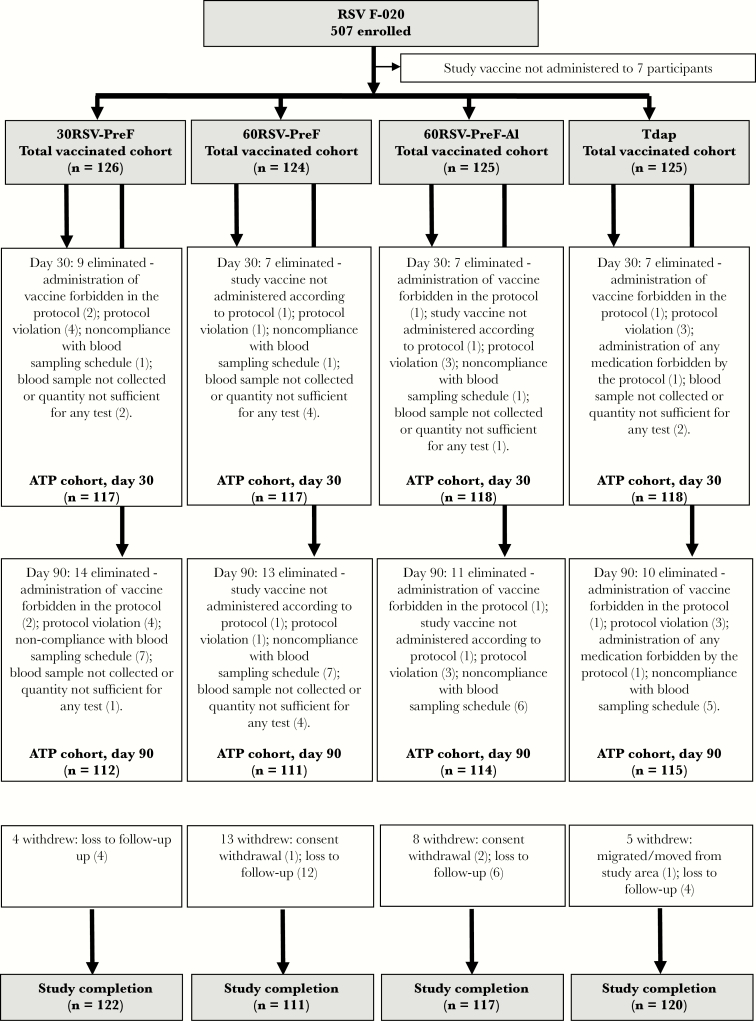
Figure 2.
Study flow. The 30RSV-PreF group received nonadjuvanted RSV vaccine containing 30 µg of RSV–prefusion F protein (PreF), the 60RSV-PreF group received nonadjuvanted RSV vaccine containing 60 µg of RSV PreF, the 60RSV-PreF-Al group received aluminum-adjuvanted RSV vaccine containing 60 µg of RSV PreF, and the Tdap group received an adult formulation of combined tetanus toxoid-diphtheria toxoid-acellular pertussis vaccine. ATP, according to protocol.