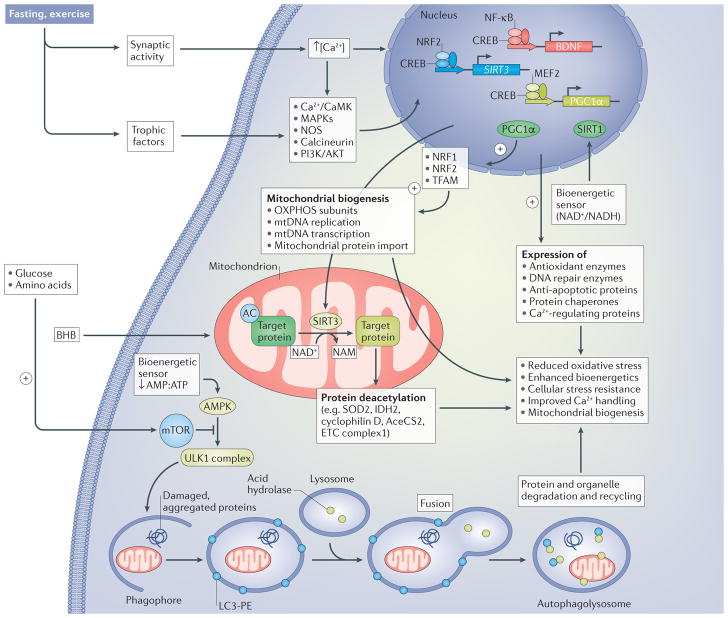
Figure 2. Signalling pathways by which neurons respond to the metabolic switch during fasting and exercise.
Increased excitatory synaptic activity triggers Ca2+ influx through plasma membrane channels, resulting in the activation of multiple kinases, including calcium/calmodulin-dependent kinases (Ca2+/CaMK) and mitogen-activated protein kinases (MAPKs), nitric oxide synthase (NOS) and the protein phosphatase calcineurin. Neurotrophic factor signalling pathways are also engaged in response to fasting and exercise, resulting in the activation of signalling pathways involving phosphatidylinositol 3-kinase (PI3K), RACα serine/threonine-protein kinase (AKT) and MAPKs. These activity-dependent and neurotrophic-factor-dependent signalling pathways converge on transcription factors, including cAMP-responsive element-binding protein (CREB), nuclear regulatory factor 2 (NRF2; also known as NFE2L2), nuclear factor-κB (NF-κB) and myocyte-specific enhancer factor 2 (MEF2), which induce the expression of many different genes that encode proteins involved in cellular stress adaptation. Three genes that may be particularly important in the enhancement of neuroplasticity and stress resistance in response to intermittent metabolic switching are those encoding brain-derived neurotrophic factor (BDNF), NAD-dependent protein deacetylase sirtuin 3 (SIRT3) and peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α). SIRT3 localizes to the mitochondria, where it deacetylates proteins involved in antioxidant defence (superoxide dismutase [Mn], mitochondrial (SOD2)), energy production (isocitrate dehydrogenase [NADP], mitochondrial (IDH2); acetyl CoA synthase 2 (AceCS2; also known as ACSS1); and electron transport chain (ETC) proteins) and protection against apoptosis (cyclophilin D). PGC1 α is a key transcriptional regulator of mitochondrial biogenesis that acts, in part, by upregulating NRF1 and mitochondrial transcription factor A (TFAM), which, in turn, upregulate oxidative phosphorylation, mitochondrial DNA (mtDNA) replication and transcription and mitochondrial protein import. CREB, NRF2 and NF-κB induce the expression of antioxidant enzymes, DNA repair enzymes, anti-apoptotic proteins and protein chaperones and Ca2+-handling proteins. The ketone β-hydroxybutyrate (BHB), which is elevated during fasting and extended exercise, provides an energy substrate for neurons and induces the expression of BDNF. The reduction in availability of glucose and amino acids during fasting and exercise results in a reduction in the AMP:ATP ratio, which activates AMP kinase (AMPK) and, in turn, stimulates autophagy. The reduction in glucose and amino acid availability also reduces activation of mammalian target of rapamycin (mTOR) and further enhances the AMPK-driven autophagy pathway. AMPK activates the serine/threonine-protein kinase ULK1 (ULK1) complex, which stimulates engulfment of damaged proteins and organelles in autophagosomes, which, in turn, fuse with lysosomes. Collectively, activation of these different pathways in response to the metabolic switch bolsters neuronal bioenergetics, improves Ca2+ handling and protects against oxidative, excitotoxic and proteotoxic stress. AC, acetyl group; LC3-PE, light chain 3– phosphatidylethanolamine; NAM, nicotinamide adenine mononucleotide; OXPHOS, ETC complexes involved in oxidative phosphorylation.