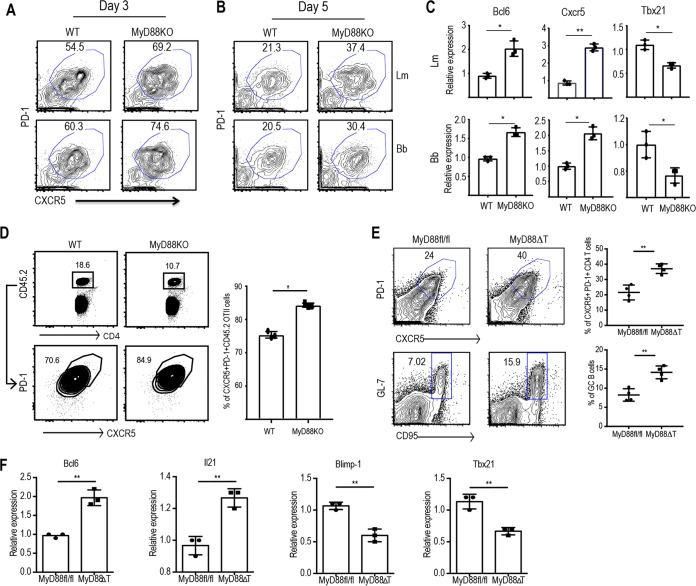
FIG 3.
T cell-intrinsic MyD88 plays a critical role in regulating the development of Tfh lineage cells. Naive CD4 T cells from WT and MyD88 KO mice were sorted and cocultured with CD11c+ splenic DCs in the presence of 10 μg/ml of L. monocytogenes or B. burgdorferi extract for 3 to 5 days. (A and B) FACS plots show the proportions of the CXCR5+ PD1+ Tfh cell populations on day 3 (A) and day 5 (B). (C) The relative expression of Bcl6, Cxcr5, and Tbx21 by sorted CFSElo CD4 T cells on day 5 upon stimulation with L. monocytogenes (top) and B. burgdorferi (bottom) extracts was quantified by qPCR. (D) Sorted naive WT OT-II cells (CD45.1) were transferred into congenic (CD45.1) immunocompetent mice (1 × 105 cells/mouse). On the next day, the mice were immunized with OVA-LPS emulsified in IFA subcutaneously in the hind footpads. A flow plot of the expanded OT-II cell population (left) and the proportion of CD4+ PD1+ CXCR5+ cells among the transferred cells (right) are shown. (E) Peyer's patches from MyD88fl/fl (control) and MyD88ΔT littermate mice were harvested, processed, and analyzed. (Left) Flow plots show the proportion of CXCR5+ PD-1+ Tfh cells (top) and CD19+ GL7+ CD95+ GC B cells (bottom) in control and MyD88ΔT mice. (Right) Quantification of flow data from five independent mice. (F) Relative expression of the respective genes in sorted CD4+ CD44hi CD62Llo T cells as quantified by qPCR. Data are representative of those from two independent experiments with three mice in each group. P values were determined by an unpaired t test. *, P > 0.05; **, P > 0.01.