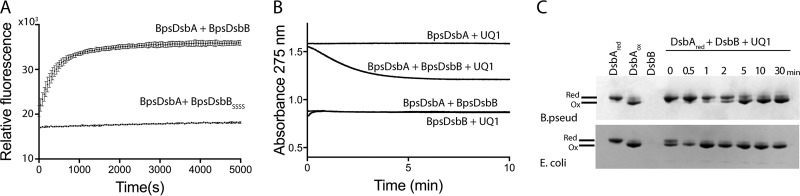
FIG 2.
BpsDsbA and BpsDsbB are redox partners. (A) BpsDsbB sustains BpsDsbA-catalyzed folding of a model peptide substrate. BpsDsbA (80 nM) and BpsDsbB (1.6 μM) (crude membrane preparation) efficiently catalyze the oxidation of a peptide substrate, as indicated by monitoring an oxidation-dependent fluorescence signal. Means and SDs are shown (n = 4). BpsDsbB in which each periplasmic cysteine residue has been mutated to serine (BpsDsbBSSSS) is unable to sustain BpsDsbA oxidative catalytic activity. Means and SDs are shown (n = 2). (B) Reduced BpsDsbA catalyzes BpsDsbB-mediated reduction of ubiquinone, as monitored by a decrease in ubiquinone absorption at 275 nm. Reaction mixtures lacking BpsDsbB, BpsDsbA, or UQ1 exhibited no A275 change. (Note that the difference in the starting absorbance arose from the different intrinsic absorptions of BpsDsbA, BpsDsbB, and mixes thereof.) The data presented are representative of those from three separate experiments. (C) Reduced (Red) BpsDsbA is converted to oxidized (Ox) BpsDsbA by a catalytic amount of detergent-solubilized purified BpsDsbB in the presence of UQ1. The reaction mixture was sampled over 30 min, and the reaction was stopped with TCA precipitation. Following AMS alkylation of free thiols (which adds 0.5 kDa to each cysteine in the reduced enzyme), reduced and oxidized BpsDsbA were separated on the basis of their different electrophoretic mobilities. Control reactions used E. coli DsbA and E. coli DsbB. The experiments were repeated on three independent occasions, and representative data from one of those experiments are reported.