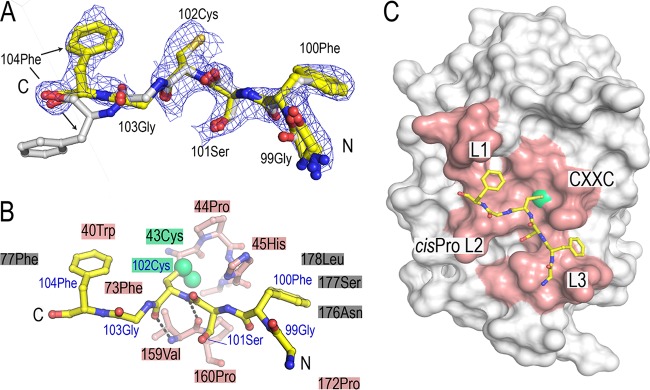
FIG 5.
Crystal structure of BpsDsbA in complex with a 6-mer peptide derived from periplasmic loop 2 of BpsDsbB (GFSCGFBpspep). (A) The conformations of the four chains in the asymmetric unit are superimposed. The binding mode is very similar in all four complexes in the asymmetric unit, with the exception of the C-terminal end of chain G (carbons colored white), where the side chain of 104 Phe is oriented away from the surface of BpsDsbA. The 2mFo − DFc map is displayed at a 1-sigma contour level for GFSCGFBpspep (chain F), where 2mFo − DFc is a sigma A weighted density map; Fo and Fc are the experimentally measured and model-based amplitudes, respectively; m is the figure of merit; and D is the sigma A weighting factor. (B) The BpsDsbB peptide is shown in stick representation and colored yellow. BpsDsbA residues forming the binding interface with the peptide are indicated with shaded boxes or shown as sticks (specifically, 43 Cys-45 His of the active-site CXXC loop and 159 Val-160 Pro of the cis-Pro loop [L2]). BpsDsbA residues which contact the peptide are colored salmon if they make up the active-site surface loops and gray if not. The active-site cysteine 43 is highlighted in green, and the sulfur atoms of both BpsDsbA 43 Cys and peptide 102 Cys are shown as green spheres. GFSCGFBpspep 102 CysBpspep makes two main-chain-mediated hydrogen bonds to cis-Pro-1 residue 159 Val of BpsDsbA, indicated by dashed black lines. (C) GFSCGFBpspep binds on the catalytic surface of BpsDsbA, engaging each of the four loop regions that comprise the active-site face of the protein: the active-site CXXC motif (43 Cys-44 Pro-45 His-46 Cys) and loops linking B3 and H2 (L1), H6 and B4 (L2), and B5 and H7 (L3) (reviewed in references 13 and 25). The BpsDsbA surface showing the region comprising the four loops is colored salmon, with the active-site cysteine (Cys 43) colored green.