Fig. 2.
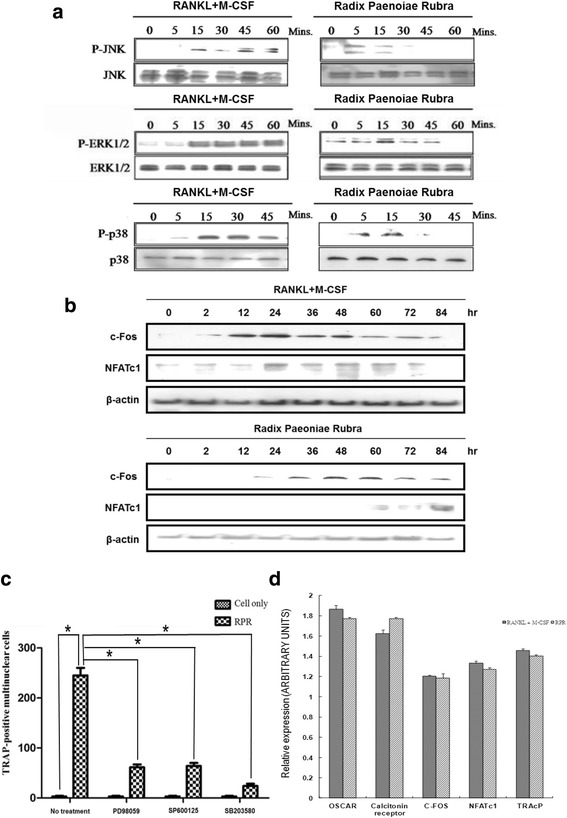
RPR-induced stimulation of MAP kinases. a RAW264.7 macrophages were subjected for specified time periods to vehicle RPR (10 μg/ml), or RANKL (50 ng/ml) and M-CSF (25 ng/ml). Cells were then solubilized, and Western blot analysis of p38, JNK, and ERK protein expression was used to examine cell lysates. The top panel of each group shows a trace that denotes the immuno-reactivity of the phosphorylated kinase. The same membrane (shown in the bottom panel) was then exposed and re-probed with the kinase antibody to identify the total kinase protein level. Outcomes represent three separate experiments. b Western blotting with Abs specific for β-actin (control), NFATc1, and c-Fos (all from Santa Cruz Biotechnology) was employed to analyze the lysates collected from cultured cells. c RPR-induced osteoclast formation required stimulation of JNK, p38, and ERK. Prior to activation with M-CSF and RANKL or RPR (10 μg/ml), RAW264.7 macrophages were pre-treated with vehicle 10 ng/ml SB203580, 10 ng/ml SP600165, or 0.5 ng/ml PD98059 for 20 mins. After culture for 7 days in RAW264.7 macrophages, a TRAP assay was employed on the cells. Data represent the mean ± SD of a minimum of three separate tests; *p < 0.01, RPR treatment versus cell only; #p < 0.01, inhibitor treatment versus no treatment. d The image shows the consequences of RANKL and RPR on osteoclast gene expression. M-CSF (200 ng/ml) and RANKL (100 ng/ml) or RPR (15 μg/ml) were applied to human monocytes. After culture for 14 days, total RNA was obtained and real-time RT-PCR was conducted for OSCAR, NFATc1, calcitonin receptors, c-fos, and TRAcP. Expression was regulated to that of β-actin; data represent the means ± SD of three repeated wells
