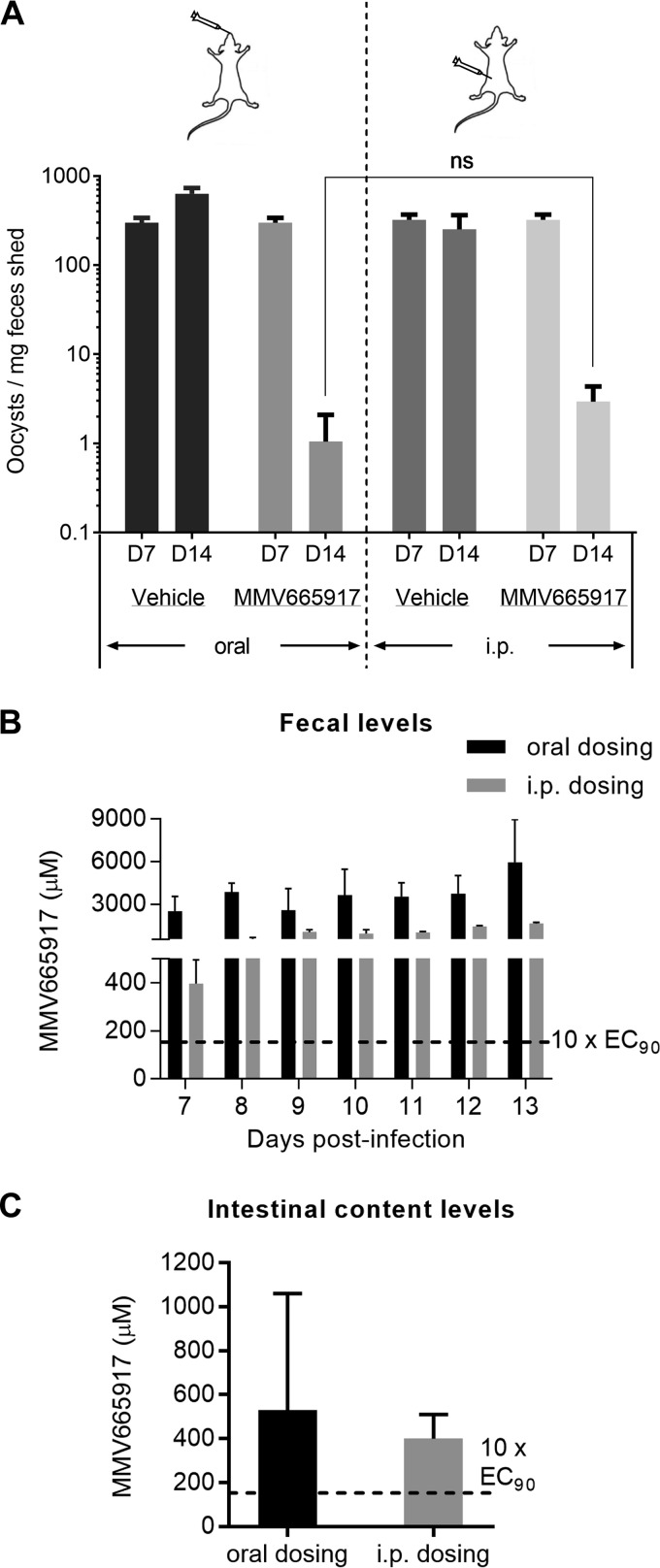
FIG 4.
Oral versus intraperitoneal (i.p.) treatment with MMV665917. (A) Efficacy measured by fecal qPCR following the administration of MMV665917 at the indicated dose by either oral gavage or i.p. injection. Efficacy was independent of the dosing route. Data are means and standard errors of the means. Four mice were used for oral dosing and three for i.p. dosing. ns, no significant difference (P = 0.2) by a Mann-Whitney test. (B) MMV665917 levels in the feces of mice treated for 7 days orally or i.p. with 60 mg/kg BID from the first 12 h of treatment and every 24 h thereafter. (C) MMV665917 levels in the small intestine following euthanasia 12 to 15 h after the last treatment dose. For panels B and C, data are expressed as micromolar concentrations of MMV665917, calculated by considering 1 g of intestinal contents or feces to be equivalent to 1 ml.