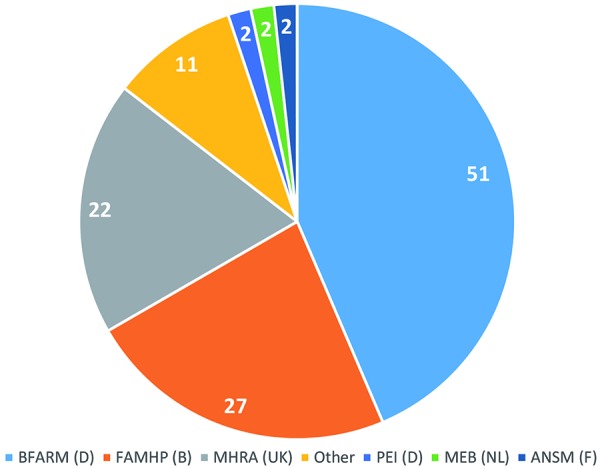
Figure 1. Distribution of submitted clinical trial applications across competent authorities (based on 123 integrated protocols, given are numbers of protocols submitted to authority). BfArM = Federal Institute for Drugs and Medical Devices, Germany (D); famhp = Federal Agency for Medicines and Health Products, Belgium (B); MHRA = Medicines and Healthcare Products Regulatory Agency, United Kingdom (UK); PEI = Paul Ehrlich Institute; Germany (D); MEB = Medicines Evaluation Board, The Netherlands (NL); ansm = National Agency for the Safety of Medicine and Health Products, France (F); Other: Irish Medicines Board, Japanese Ministry of Health and Welfare, Hungarian Authority.