Figure 1. Iron metabolism in the human body.
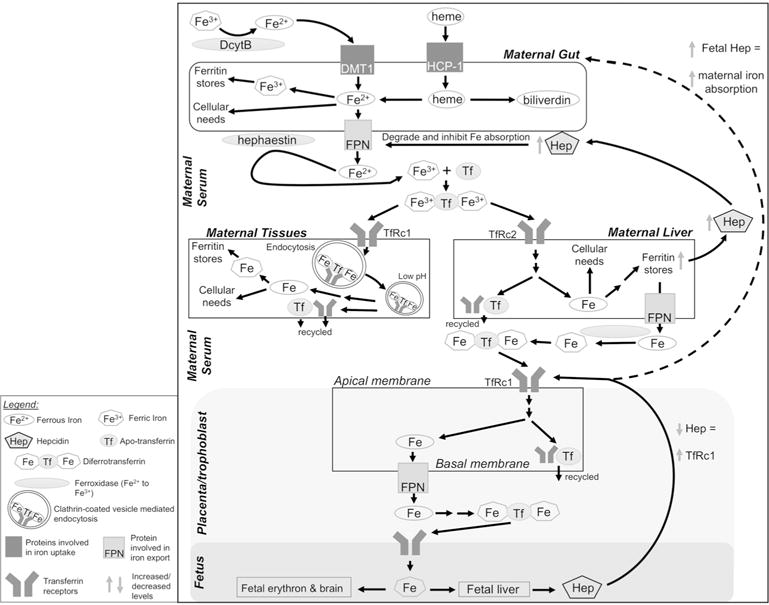
Upper panel: Dietary non-heme iron (Fe3+) is reduced to Fe2+ via duodenal cytochrome b (DcytB) and imported into enterocytes by divalent metal ion transporter-1 (DMT1). Heme is imported via heme-carrier protein-1 (HCP-1). In the enterocyte, Fe2+ may be used as a protein cofactor, oxidized to Fe3+ for storage in ferritin, or exported from the cell by ferroportin (FPN). Enterocyte-bound hephaestin oxidizes Fe2+, and two Fe3+ bind apotransferrin (Tf) to form diferrotransferrin in the serum. Middle panel: Target tissues, including liver, present transferrin receptors (TfRc1 or TfRc2) and acquire diferrotransferrin via endocytosis of clarithin-coated vesicles. Decreased vesicular pH releases Fe3+, which is reduced to Fe2+ via metalloreductase STEAP3 and transported out of the vesicle by DMT1 (not shown). Intracellular Fe2+ is used as a protein cofactor or stored in ferritin. Apo- transferrin and TfRc are recycled to the cell surface. Increased liver iron upregulates hepatic hepcidin (Hep) production. Hep causes FPN degradation to decrease iron absorption. Lower panel: During pregnancy, TfRc1 on the apical membrane of placenta/trophoblast enables fetal iron transfer. Diferrotransferrin is internalized as above and the iron is stored, utilized, or transported across the basal membrane via FPN. Iron enters fetal circulation for utilization and distribution to target tissues. Low fetal Fe leads to low hepcidin which signals increased placental TfRc1; fetal hepcidin may also increase iron absorption in maternal enterocytes, but the underlying mechanism is unknown (indicated by dashed arrow). DcytB, duodenal cytochrome b; DMT1, divalent metal ion transporter-1; Fe, iron; FPN, ferroportin; HCP-1, heme-carrier protein-1; Hep, hepcidin; Tf, transferrin; TfRc1/2, transferrin receptor-1 or -2.
