Scheme 1. General synthetic routes for generating analogs.
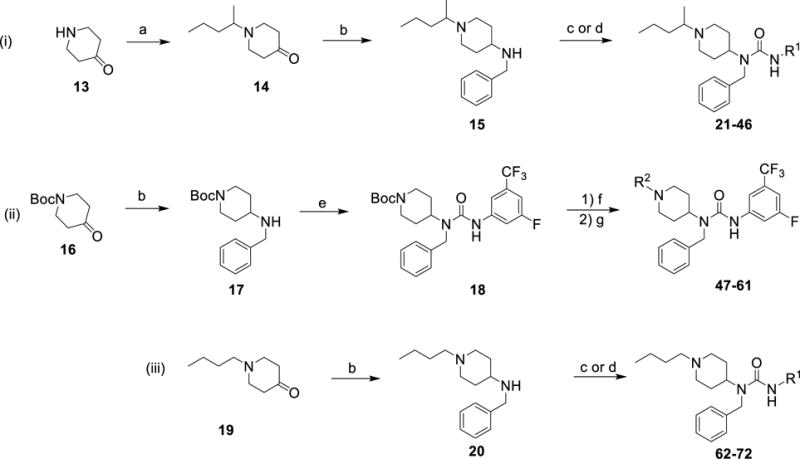
(a) potassium carbonate, 2-iodopentane, acetonitrile, 80 °C, 16 h, 30-48% (b) benzylamine, sodium triacetoxyborohydride, acetic acid, dichloromethane, rt, 16 h, 90-100%; (c) R1-isocyanate, N,N-diisopropylethylamine, dichloromethane, rt, 2 h, 40-80%; (d) R1-amine, 1,1′-carbonyldiimidazole, tetrahydrofuran, 16 h, 30-55%; (e) 3-Fluoro-5-(trifluoromethyl)phenyl isocyanate, N,N-diisopropylethylamine, dichloromethane, rt, 2 h, 80-99%; (f) trifluoroacetic acid, dichloromethane, 0 °C - rt, 16 h, 95%; (g) R2 (aldehyde or ketone), dibutyltin (IV) chloride, phenylsilane, tetrahydrofuran, rt, 16 h, 40-75%.
