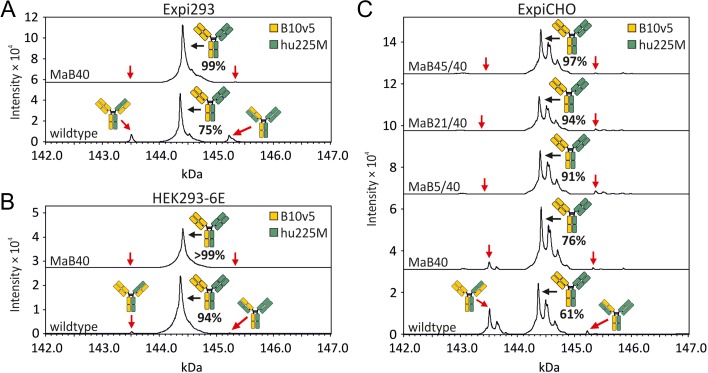
Fig. 6.
Analysis of pairing of light to heavy chain in the bsAb B10v5 × hu225M by LC–ESI–MS, after protein A purification and deglycosylation. The bsAbs contained either no mutation in the Fab interface (wildtype) or contained mutations in the CH1:CL interface (see Table II for a complete list). The VH:VL interface was left unmutated in all cases. The antibodies were produced by transient transfection of (A) Expi293, (B) HEK293-6E or (C) ExpiCHO. The prevalence of correctly assembled bsAb is given in percent of all detected heterodimeric IgGs and represents a mean of two (A) or three (B and C) independent transfections (Table I).