Fig. 1.
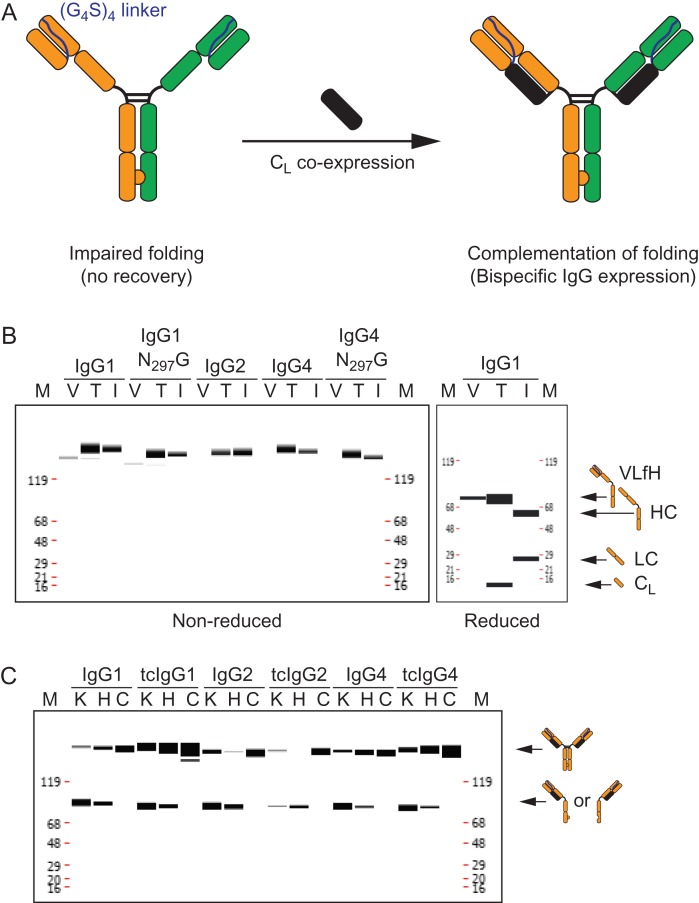
tcBsIgG format enables bispecific antibody generation in a single cell line. (A) Schematic representation of the tcBsIgG format. The antibody VL domain is tethered via a (G4S)4 linker to the antibody heavy chain for the VLfH format (left). The folding defect of CH1 is complemented by CL expression in trans from a separate plasmid (right). (B) Capillary electrophoresis under non-reducing (left panel) and reducing conditions (right panel) of expression of IgG1, IgG2, and IgG4 as well as the aglycosylated (N297G) version of IgG1 and IgG4 after protein A affinity column purification recovery. Expression as VLfH alone (lane V) without the CL in HEK293 cells results in only trace amount of or no VLfH expression at all; VLfH co-expressed with CL (lane T) results in tcIgG expression that is comparable to a standard IgG expression (lane I). (C) Capillary electrophoresis of protein A affinity purified tcIgG for conventional IgG1, IgG2, and IgG4 as well as the tcIgG1, tcIgG2, and tcIgG4 counterparts as knob half-antibody (lane K), hole half-antibody (lane H), and knob and hole co-expressed IgG in a single cell (lane C).