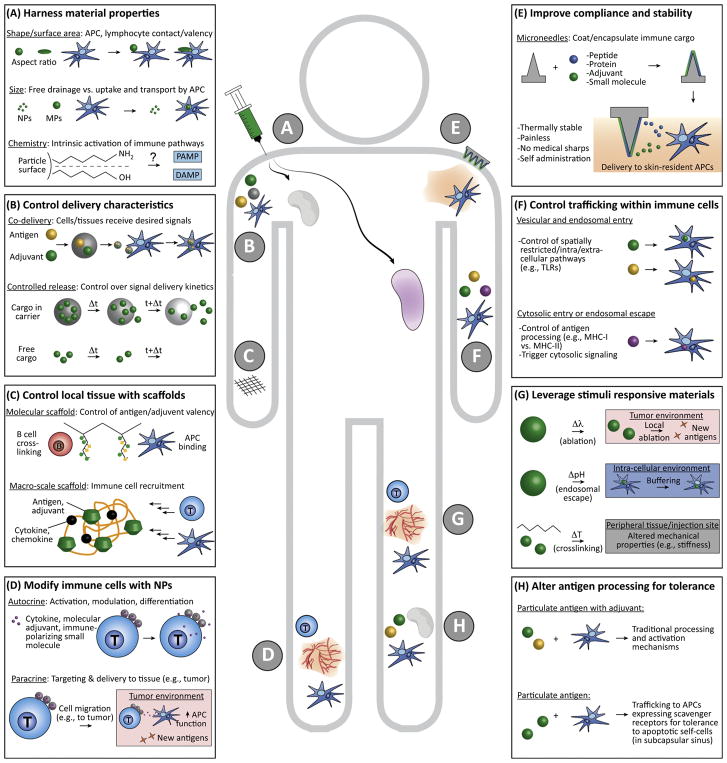
Figure 2. Strategies Involving Biomaterials for Engineering Immune Function.
(A) Material shape, size, chemistry, and other physicochemical properties impact drainage through lymphatics, interactions with APCs, and the intrinsic immunogenicity of many common polymers. (B) Biomaterials can be used to control the combinations and relative concentrations of immune cargos reaching APCs and lymphocytes, or, by designing polymers with a desired degradation rate, the cargo delivery kinetics. (C) Scaffolds can be used to control the context or density in which antigens and adjuvants are displayed, and as local environments to recruit APCs or lymphocytes (e.g., by incorporation of chemokines). (D) T cells, APCs, and other immune cells can be modified with nanoparticles incorporating immune signals to exert autocrine effects on the modified cells, or to exert paracrine effects on target cells and tissue to which the modified cell will migrate (e.g., a tumor). (E) Microneedles coated with or incorporating immune cues increase safety and patient compliance by efficiently targeting skin-resident immune cells without pain, generation of medical sharps, or need for refrigeration. (F) Biomaterial carriers can be engineered with specific moieties to control cellular entry and intracellular trafficking for directing spatially restricted immune processes (e.g., TLR signaling and antigen processing). (G) Stimuli responsive materials can exploit physiological (e.g., changes in pH or temperature) or external cues (e.g., UV light) to provide environment-specific control within cells, target tissues, or tumors (e.g., access to neoantigens during NP-enabled local ablation via photothermal exposure). (H) NPs and MPs can alter how antigens are processed to modulate responses away from proimmune and toward regulation. Abbreviations: APC, antigen-presenting cell; MP, microparticle; NP, nanoparticle; TLR, Toll-like receptor.