Figure 2. Treatment Modalities for Immunotherapy.
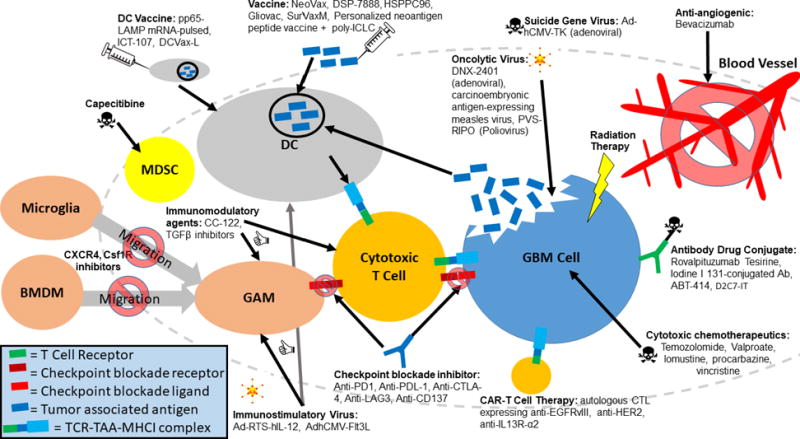
Immunotherapeutics are designed to exert their effects on various cell types within the glioma microenvironment. Anti-angiogenics block neovascularization of gliomas. Radiation and cytotoxic chemotherapeutics exert their effects on rapidly dividing glioblastoma (GBM) cells by causing irreparable DNA damage and/or inhibiting vital cellular processes. Antibody drug conjugates deliver cytotoxic chemotherapeutics to cells with higher specificity by targeting tumor associated antigens (TAAs). Oncolytic viruses and viruses carrying suicide genes are targeted to GBM cells overexpressing particular receptors and cause immunogenic cell death. Chimeric antigen receptor (CAR) T cells are engineered to elicit efficient killing against cells expressing specific TAAs. Checkpoint blockade inhibitors prevent T cell anergy by blocking inhibitory interactions between T cells and target cells. CXCR4 and Csf1R inhibitors block bone marrow derived macrophages (BMDMs) and microglia from migrating to tumors. Capecitibine depletes immunosuppressive MDSCs (myeloid derived suppressor cells). Immunomodulatory agents and immunostimulatory viruses enhance anti-tumorigenic polarization of various immune cells within the glioma microenvironment. Vaccines and dendritic cell (DC) vaccines serve to elicit potent anti-tumor effects by education and stimulation of anti-TAA cytotoxic T cells. GAM = glioma associated microglia/macrophage.
