Beginning in the 1950's, Arthur Kornberg's research on DNA polymerases, followed by the efforts of many others, provided a seminal understanding of catalytic DNA synthesis [1], a fundamental process enabling the anti-parallel strands of the DNA double helix to be copied by DNA polymerases with a single polarity. A leading strand, copied in a relatively continuous manner, and a lagging strand, copied in a discontinuous manner as a series of Okazaki fragments, resolved the issue. Building upon this work, Bruce Alberts proposed the “trombone model” over 35 years ago to visualize the apparent coordination between leading- and lagging-strand synthesis at the replication fork [2].
Although the emerging story of coordinated semi-conservative DNA replication is satisfying, it is now evident that during conditions of replication stress (invoked by nucleotide depletion, DNA damage, secondary DNA structure, oncogene activation, etc.), the scenario is more complex than previously thought. Fork dynamics and the cellular response dictate sophisticated mechanisms to ensure fork integrity, resumption of DNA synthesis, and ultimately genomic stability. During replication stress, the DNA replication checkpoint response mediated by apical kinases (S. cerevisiae Mec1, human ATR) and effector kinases (S. cerevisiae Rad53, human CHK1) adjusts cell cycle transitions (including entry into mitosis), suppresses firing of new replication origins, elevates the deoxynucleotide (dNTP) pool, and modulates gene gating proposed to relieve topological stress [3]. In addition, replication stress may directly affect replication fork DNA architecture through remodeling events and by altering interactions of proteins that stably or transiently associate with the replisome [3]. These aspects of fork dynamics are still not well understood.
A very recent study by Zhiguo Zhang of Columbia University and colleagues, appearing in the October 19, 2017 issue of Molecular Cell, has provided a fresh perspective on how the DNA replication checkpoint pathway plays a role in the coordination of leading- and lagging-strand synthesis during hydroxyurea (HU)-induced replication stress which depletes the dNTP pool [4]. Using a clever experimental strategy to measure nascent leading and lagging strands in wild-type and rad53-1 mutant checkpoint defective yeast cells, the authors convincingly show that a deficiency in the replication checkpoint results in asymmetric DNA synthesis with extended DNA synthesis complementary to the lagging-strand template, but not the leading-strand template. Concurrently, the exposed single-stranded DNA present in the leading-strand template is coated by Replication Protein A (RPA), which normally provides an early signal for the replication checkpoint. In rad53-1 checkpoint defective cells, the replication fork helicase known as minichromosome maintenance protein complex (MCM) and associated DNA polymerase epsilon (ε) move ahead of the newly synthesized DNA (without continuing to catalyze polynucleotide synthesis), which is likely to further contribute to fork asymmetry under the condition of HU-induced replication stress. Finally, the authors provide evidence that increasing the dNTP level in the HU-treated rad53-1 mutant cells effectively suppressed the uncoordinated leading- and lagging-strand synthesis.
The significance of this work lies in the demonstration that eukaryotic cells rely on a checkpoint mechanism to prevent uncontrolled asymmetric DNA synthesis at the fork, presumably by inhibiting MCM-catalyzed unwinding of the parental duplex. However, the suppressive effect of activated Rad53 on MCM helicase activity was not formally shown in the study. Nonetheless, this work provides new insight to a cellular pathway whereby replication fork catastrophe is avoided and genomic integrity is maintained, even under adverse conditions of compromised DNA synthesis. However, like many innovations, this study raises some important unanswered questions pertaining to the mechanism whereby eukaryotic cells ensure coordinated leading- and lagging-strand synthesis during replication stress (Figure 1).
Figure. 1.
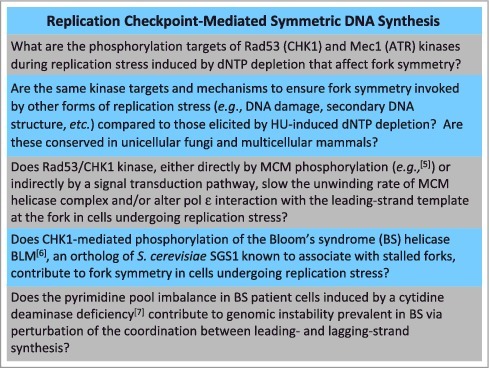
A recent advance by Gan et al. [4] on the role of the replication checkpoint in coordinating leading- and lagging-strand synthesis during replication stress raises new and provocative questions in the field.
Although there has been a plethora of studies on the S-phase checkpoint and pathways to maintain the replication fork and ensure chromosomal stability, truly mechanistic insights have been lagging (so to speak) in the field. This latest advance by Gan et al. [4] should inspire new efforts and innovative experimental strategies to characterize the precise molecular pathways responsible for promoting coordinated DNA synthesis of the leading- and lagging-strands during replication in vivo, a process so fundamental to DNA biology but still difficult to fully comprehend.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- [1].Lehman IR. Discovery of DNA polymerase. J Biol Chem. 2003;278:34733–34738. doi: 10.1074/jbc.X300002200. PMID:12791679 [DOI] [PubMed] [Google Scholar]
- [2].Sinha NK, Morris CF, Alberts BM. Efficient in vitro replication of double-stranded DNA templates by a purified T4 bacteriophage replication system. J Biol Chem. 1980;255:4290–4293. PMID:6989836 [PubMed] [Google Scholar]
- [3].Giannattasio M, Branzei D. S-phase checkpoint regulations that preserve replication and chromosome integrity upon dNTP depletion. Cell Molec Life Sci. 2017;74:2361–2380. doi: 10.1007/s00018-017-2474-4. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gan H, Yu C, Devbhandari S, et al. Checkpoint kinase Rad53 couples leading- and lagging-strand DNA synthesis under replication stress. Mol Cell. 2017;68:1–10. doi: 10.1016/j.molcel.2017.09.018. PMID:29033319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Han X, Mayca Pozo F, Wisotsky JN, et al. . Phosphorylation of minichromosome maintenance 3 (MCM3) by checkpoint kinase 1 (Chk1) negatively regulates DNA replication and checkpoint activation. J Biol Chem. 2015;290:12370–12378. doi: 10.1074/jbc.M114.621532. PMID:25809478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kharat SS, Tripathi V, Damodaran AP, et al. . Mitotic phosphorylation of Bloom helicase at Thr182 is required for its proteasomal degradation and maintenance of chromosomal stability. Oncogene. 2016;35:1025–1038. doi: 10.1038/onc.2015.157. PMID:26028025 [DOI] [PubMed] [Google Scholar]
- [7].Chabosseau P, Buhagiar-Labarchède G, Onclercq-Delic R, et al. . Pyrimidine pool imbalance induced by BLM helicase deficiency contributes to genetic instability in Bloom syndrome. Nat Comm. 2011;2:368–373. doi: 10.1038/ncomms1363. PMID: [DOI] [PubMed] [Google Scholar]


