Figure 4.
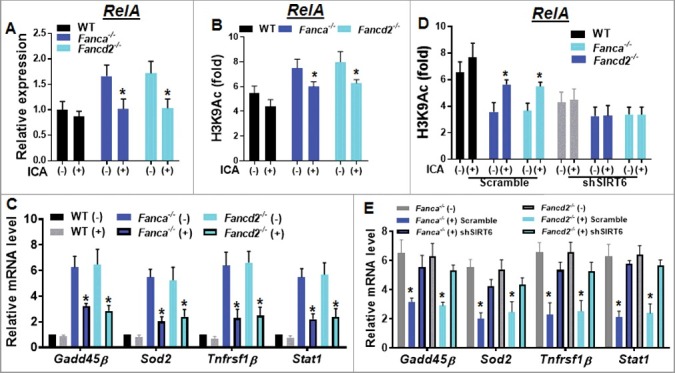
Upregulated SIRT6 by ICA inhibits NF-κB in FA HSPCs. (A) Higher RelA expression in Fanca−/− and Fancd2−/− HSPCs. RNA were extracted from Lin− cells isolated from ICA treated or untreated WT, Fanca−/− or Fancd2−/− mice followed by quantitative PCR analysis using primers for RelA (Table S2). Samples were normalized to the level of GAPDH mRNA. (B) ICA enhances the deacetylation of histone H3 lysine 9 (H3K9) on the promoter of NF-κB (RelA). Cells described in (A) were subjected to chromatin immunoprecipitation using α-H3K9Ac and α-H3 antibodies. H3K9 acetylation at RelA gene promoter is shown relative to untreated control samples and normalized to total H3 levels. (C) ICA represses NF-κB target gene expression. RNA was extracted from LSK cells described in (A) and subjected to qPCR analysis using primers listed in Table S2. Samples were normalized to the level of GAPDH mRNA. (D) The enhanced deacetylation of H3K9 on the promoter of NF-κB by ICA is mediated by upregulation of SIRT6. Lin− cells isolated from Fanca−/−, Fancd2−/− and their WT littermates were transduced with lentiviral vector expressing shRNA targeting SIRT6 or control sequence. Sorted GFP+ cells were chromatin immunoprecipitated with α-H3K9Ac and α-H3 antibodies. H3K9 acetylation at RelA gene promoter is shown relative to untreated control samples and normalized to total H3 levels. (E) Knockdown SIRT6 abolishes ICA-induced repression of NF-κB target genes. RNA was extracted from GFP+ cells described in (D) followed by qPCR using primers listed in Table S2. Samples were normalized to the level of GAPDH mRNA. Results are means ±SD of 3 independent experiments (n = 3).
