Figure 3.
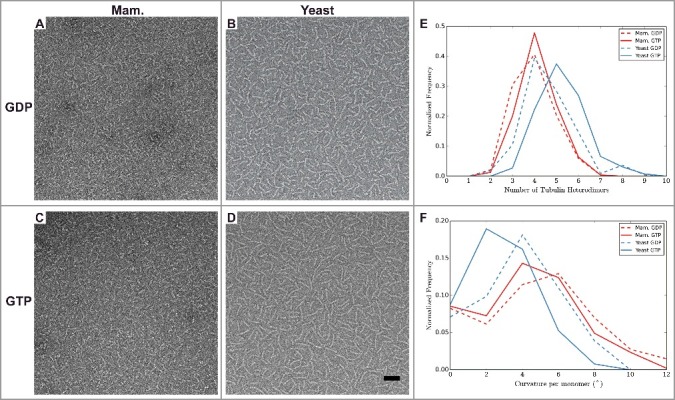
Analysis of tubulin oligomers reveals differences outside the MT lattice that may contribute to distinct dynamic instability properties of mammalian and yeast MTs. A-D) Negative stain images of (A-B) GDP and (C-D) GTP bound oligomers for (A and C) mammalian, and (B and D) yeast tubulin sources. Quantification of oligomer length (E) and curvature (F) for manually traced oligomers. The number of tubulin dimers in the mammalian oligomers does not change with nucleotide, unlike for the yeast oligomers, which are longer when GTP-bound. The majority of yeast oligomers are less curved when GTP-bound than when GDP-bound, unlike the mammalian tubulin (Mann-Whitney test p < 1 × 10−5 between mammalian and yeast GTP-bound oligomers, and Mann-Whitney test p < 1 × 10−3 between mammalian and yeast GDP-bound oligomers). Scale bar 50 nm. n > 200 for each condition.
