Figure 8.
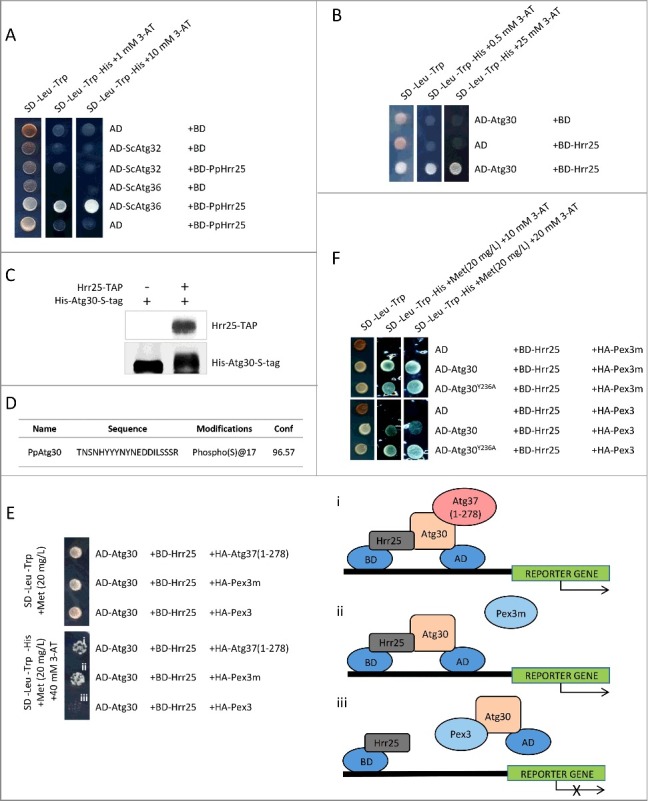
Hrr25 phosphorylates Atg30 and Pex3 inhibits the interaction of Atg30 with Hrr25. (A) Hrr25 from P. pastoris interacts with the pexophagy receptor, Atg36, but not with the mitophagy receptor, Atg32, from S. cerevisiae in a Y2H assay; BD-PpHrr25 was used with the previously described AD-ScAtg36 and AD-ScAtg32.9 (B) Atg30 interacts with Hrr25 in a Y2H assay; AD-Atg30 was used with BD-Hrr25. (C) Atg30 mobility shift on an SDS-PAGE gel after in vitro phosphorylation with Hrr25 kinase. Proteins were visualized with anti-calmodulin-binding peptide and anti-HIS tag antibodies; (D) Atg30 is phosphorylated at S112 by Hrr25 in vitro; MS2 data revealing the S112 phosphopeptide. (E) Pex3, but not Pex3m or Atg37, affects Atg30 interaction with Hrr25 in a Y3H assay. Expression of HA-Atg37(1-278), HA-Pex3 or HA-Pex3m was controlled by the MET25 promoter and a low level of methionine in the medium (20 mg/L) was used to express moderate levels of these proteins. i. Schematic depicting interaction between BD-Hrr25 and AD-Atg30 in the presence of HA-Atg37(1-278) in a Y3H assay. Binding of Atg37(1-278) to AD-Atg30 does not affect Atg30-Hrr25 complex formation and, therefore, reporter gene expression. ii. Schematic depicting interaction between BD-Hrr25 and AD-Atg30, which is unaffected by the presence of HA-Pex3m in the Y3H assay. iii. Schematic depicting depletion of interaction between BD-Hrr25 and AD-Atg30 in the presence of HA-Pex3 in the Y3H assay. (F) Disruption of the Pex3-binding site located in close proximity to the Atg37-binding site in Atg30 restores recruitment of Hrr25 to Atg30 even in the presence of Pex3 in a Y3H assay; AD-fusions of WT Atg30 or its mutants (Atg30Y236A, Atg30L241A and Atg30Y244A) were used with BD-Hrr25. Expression of HA-Pex3 or HA-Pex3m was controlled by the MET25 promoter.
