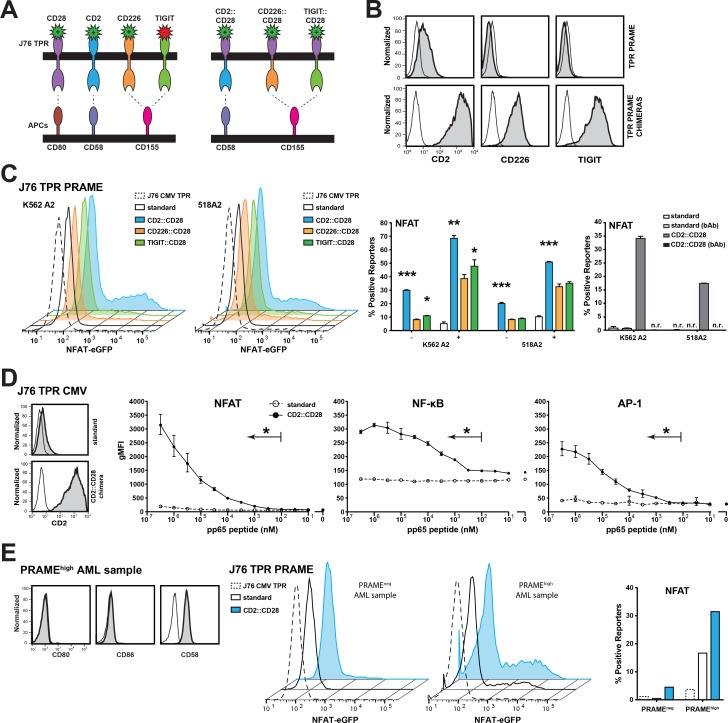
Figure 4. Chimeric CD28 receptors boost TPR sensitivity.
(A) Schematic illustration of the generated chimeric CD28 receptors. (B) Expression analysis of the chimeric CD28 receptors (grey) or appropriate isotype control (open) on J76 TPR PRAME using flow cytometry. (C) Unloaded (–) or 100 nM peptide loaded (+) K562-based engineered APCs (eAPC) and 518A2 melanoma cells were used to evaluate the potential of the chimeric CD28 receptors. Depicted histograms show NFAT activation of different PRAME TPRs by endogenous PRAME antigen presentation. J76 TPR CMV CD2::CD28 is shown as negative control. Color of histograms and bars correspond to colors of chimeric receptors depicted in (A). Right panel: A CD58 blocking antibody (bAb; 10 μg/mL) was used to confirm the specific contribution of the CD2::CD28 chimera; n.r. no reactivity. (D) J76 CMV TPR were equipped with the CD2::CD28 chimera (left). The sensitivity of the resulting reporter and the standard CMV reporter to stimulation with K562 HLA-A2+ cells loaded with antigenic peptide at different concentrations was determined (right). Geometric mean flourescent intensity of reporters is shown for duplicate values and experiment is representative of three independent experiments. (E) A primary AML sample that showed high PRAME expresssion was tested for expression of CD80, CD86 and CD58 (left). J76 CMV TPR (negative control), J76 PRAME TPR and J76 PRAME TPR CD2::CD28 were cocultured with PRAMEneg and PRAMEhigh AML samples and NFAT reporter responses are depicted (right). Statistical analysis was performed using Wilcoxon (C) and Friedman (D) tests. Differences to standard reporters are shown (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).