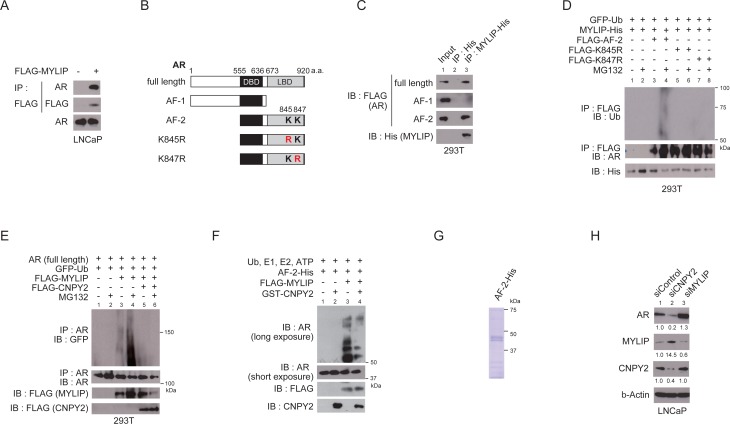
Figure 2. CNPY2 inhibits MYLIP-mediated AR ubiquitination and protein degradation.
(A) Immunoprecipitation of LNCaP cell extracts with anti-FLAG M2 affinity gel. LNCaP cells were transfected with FLAG-tagged MYLIP expression plasmids for 24 h and used for the immunoprecipitation. AR bound to MYLIP was then detected by immunoblotting. (B) Diagrams representing AR protein structure. K845 (Lys 845) and K847 (Lys847) are the two conserved ubiquitination sites on AR. DBD, DNA binding domain. LBD, Ligand binding domain. (C) Immunoprecipitation of 293T cell extracts with anti-His tag affinity beads. 293T cells were transfected with FLAG-tagged AR (full length, AF-1 or AF-2) expression plasmids and MYLIP-His or His-tag expression plasmids for 24 h and used for the immunoprecipitation. FLAG-ARs bound to MYLIP-His were then detected by immunoblotting with anti-FLAG. (D) MYLIP mediated-ubiquitination of AR was detected by in vivo ubiquitination assay. 293T cells were transfected with FLAG-AR (AF-2, K845R or K847R), MYLIP-His and EGFP-ubiquitin expression plasmids for 24 h and 10 µM MG132 was added to the culture medium 5 h before cell extraction. Cells were lysed and subjected to immunoprecipitation using anti-FLAG M2 affinity gel, followed by immunoblotting with each antibody. (E) In vivo ubiquitination assays were performed using 293T cells transfected with plasmids as indicated. Immunoprecipitation of AR (full length) was done using anti-AR (N-20). (F) In vitro ubiquitination assays were performed using recombinant AR (AF-2)-His, recombinant GST-CNPY2 and immunoprecipitated with FLAG-MYLIP. Reactions were performed with recombinant E1 enzyme, E2 enzyme and ubiquitin at 37° C for 2 h. Ubiquitination of AR was detected by immunoblotting with anti-AR (C-19). (G) Coomassie Brilliant Blue staining with recombinant AR (AF-2)-His protein. (H) Immunoblots using CNPY2 or MYLIP-knockdown LNCaP cell lysates with anti-AR, anti-MYLIP, or anti-CNPY2 antibodies. Band intensity was quantified by Adobe Photoshop. The measurements were normalized to si-Control protein levels that are indicated at the bottom of each band.