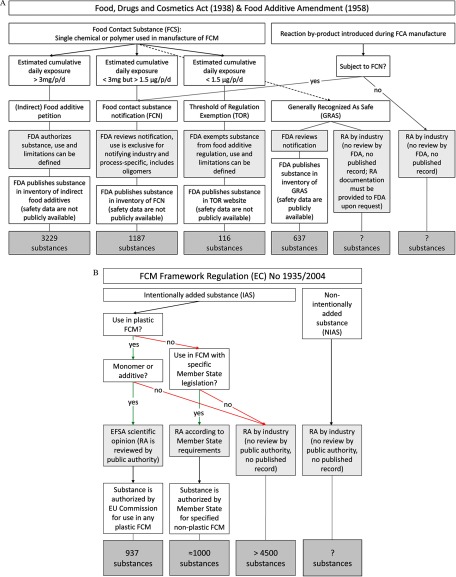
Figure 2.
Risk assessment stakeholder roles for FCCs in United States (A) and European Union (B). (A): In the United States, different rules apply to those FCCs that are food contact substances (FCS) (i.e., single chemicals or polymers used in the manufacture of FCMs), depending on their CEDI per person (p) and day (d) (Food Additive Amendment of 1958). Substances in the FDA’s GRAS inventory may not all be FCSs, as direct food additives can also be notified as GRAS. Further details of U.S. legislation on indirect food additives are provided in the Supplemental Material. (B): In the European Union, starting substances, such as monomers, additives, catalysts and some production aids are considered IAS. Impurities, by-products from manufacturing processes, and degradation products are NIAS (EC 2011). Overall, 17 different FCMs are defined in the European Union (EC 2004); currently, four FCMs are regulated with E.U.-wide specific measures issued by the European Commission, which are binding in all E.U. member states (active and intelligent materials; plastics, including recycled plastic; regenerated cellulose; and ceramics, which do not require FCC authorization). The remaining 13 FCM types may have material-specific European Union member-state regulations in place (Simoneau 2016), which are not issued by the European Commission (adhesives, cork, glass, ion-exchange resins, metals and alloys, paper and board, printing inks, rubbers, silicones, textiles, varnishes and coatings, waxes, and wood). Further details are provided in the Supplemental Material.