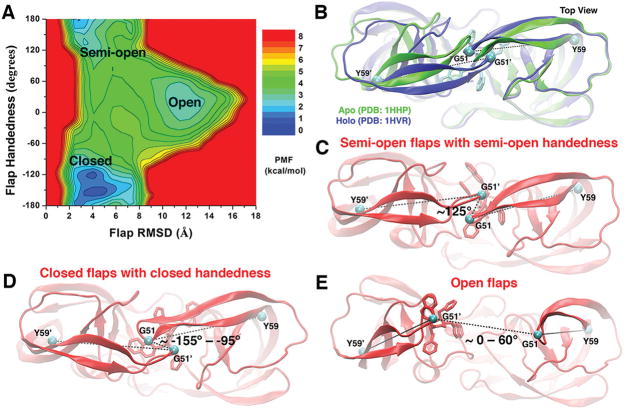
Figure 3.
(A) 2D PMF profile of the protein flap RMSD and handedness calculated by combining the “Sim1” and “Sim2” GaMD trajectories, during which the XK263 ligand molecule was observed to bind the protein active site. Three low-energy conformational states of the protein flaps are labeled, including the “open”, “semi-open”, and “closed” states. (B) Top view of the X-ray crystal structures of the HIV protease in the apo (PDB entry 1HHP, green) and XK263-bound holo (PDB entry 1HVR, blue) forms. The ligand is shown as sticks and the protein as ribbons. The dihedral angle of the Cα atoms (cyan spheres) of the Tyr59-Gly51-Gly51′-Tyr59′ motif is used to characterize the flap handedness. (C–E) Conformational states of the HIV protease: “semi-open” flaps with “semi-open” handedness, “closed” flaps with “closed” handedness, and “open” flaps, respectively. The evolving protein is shown as ribbons and the ligand molecule as sticks.