Table 1.
Optimization of the Aldehyde C–H Arylation.a
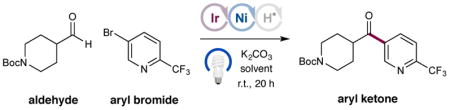
| |||
|---|---|---|---|
| entry | conditions | solvent | yieldb |
| 1 | as shown | DMSO | 2% |
| 2 | as shown | CH3CN | 8% |
| 3 | as shown | dioxane | 92% (87%) |
| 4 | 2 mol% Ni catalyst | dioxane | 80% |
| 5 | no photocatalyst | dioxane | 0% |
| 6 | no Ni catalyst | dioxane | 0% |
| 7 | no light | dioxane | 0% |
Photocat 1 (1 mol%), NiBr2·dtbbpy (10 mol%), quinuclidine (10 mol%), aryl halide (1.0 equiv), aldehyde (2.0 equiv), and K2CO3 (1.5 equiv). Yield by 1H NMR analysis.
Isolated yields in parentheses.
