Abstract
Objectives
In some individuals with obstructive sleep apnea (OSA), the palate prolapses into the velopharynx during expiration, limiting airflow through the nose or shunting it out the mouth. We hypothesized that this phenomenon causes expiratory flow limitation (EFL) and is associated with inspiratory “isolated” palatal collapse. We also provide a robust non-invasive means to identify this mechanism of obstruction.
Methods
Using natural sleep endoscopy, 1211 breaths from 22 OSA patients were scored as having or not having palatal prolapse. The patient-level site of collapse (tongue-related, isolated palate, pharyngeal lateral walls, and epiglottis) was also characterized. EFL was quantified using expiratory resistance at maximal epiglottic pressure. A non-invasive expiratory flow limitation index (EFLI) was developed to detect the presence of palatal prolapse and EFL using the flow signal alone. In addition, the validity of using nasal pressure was assessed.
Results
A cutoff value of EFLI>0.8 detected the presence of palatal prolapse and EFL with an accuracy of >95% and 82%, respectively. The proportion of breaths with palatal prolapse predicted isolated inspiratory palatal collapse with 90% accuracy.
Conclusions
This study demonstrates that expiratory palatal prolapse can be quantified non-invasively, is associated with EFL, and predicts the presence of inspiratory isolated palatal collapse.
Keywords: Palatal prolapse, Isolated palatal collapse, Expiratory Flow Limitation, Uvula, Nasal pressure, Sleep Apnea
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disorder characterized by recurrent upper airway collapse during sleep(1), which causes sleep fragmentation(2) and sympathetic activation(3). Untreated OSA has also been associated with adverse neurocognitive (daytime sleepiness, reduced attention)(4–6) and cardio-metabolic complications (hypertension, diabetes, strokes)(4, 7, 8).
In patients with OSA, upper airway obstruction is caused by collapse at one or more pharyngeal sites during sleep (palate, tongue base, lateral walls, epiglottis)(9, 10). In the course of existing investigations by our group to identify these structure(s) endoscopically, we observed that, in some individuals, the soft palate and uvula prolapsed (ballooned) into the velopharynx during expiration (Figure 1). These instances were easy to recognize because they produced substantial pharyngeal narrowing/fluttering and occasional redirection of expiratory flow out the mouth (unidirectional airflow – breathing in through the nose and out through the mouth). The movement of the involved floppy tissues (soft palate and uvula) was often quite dramatic and caused arousals.
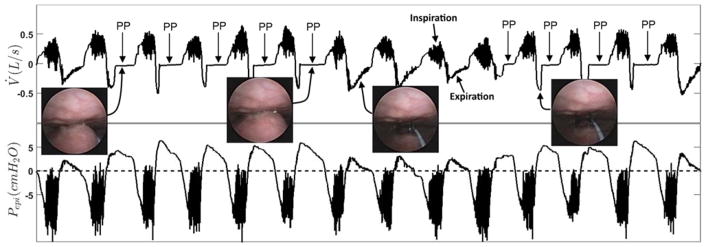
Figure 1.
Protrusion or “ballooning” of the palate into the velopharynx during expiration (palatal prolapse (PP)). The two endoscopic images on the left displays the palatal prolapse from the velopharyngeal view of the airway during expiration in two different breaths. The third endoscopic image from the left shows an open airway during normal expiration. The right endoscopic image also displays an open airway just before the occurrence of the palatal prolapse. The top panel shows the pneumotachograph-measured airflow (V̇) and the bottom panel demonstrates the simultaneously measured epiglottic pressure (Pepi). This figure clearly shows a distinct and reproducible expiratory flow pattern whenever the palate prolapses (balloons) into the velopharynx. Expiratory flow limitation (EFL) is observed from the lack of increase in expiratory flow despite an increase in epiglottic pressure during a period with repeated palatal prolapse (arrows identified with PP). During palatal prolapse, expiratory positive airway pressure develops at the level of the epiglottis and is not associated with a corresponding increase in the expiratory nasal flow.
In addition, research shows that expiratory flow limitation (EFL), which is coupled with inspiratory flow limitation in 50% of the breaths(11), is a common clinical finding(11–14) and could potentially be used to predict obstructive pulmonary disease(15) and the efficiency of mask ventilation during anesthesia induction(16) in patients with sleep disordered breathing. However, the mechanism of action remains unknown.
We hypothesized that EFL is typically caused by palatal prolapse (PP). Therefore, we analyzed endoscopy videos recorded simultaneously with flow and epiglottic pressure during natural sleep to examine this hypothesis. Furthermore, we hypothesized that palatal prolapse produces well-defined, reproducible intra-breath expiratory airflow characteristics during sleep that can be automatically identified from expiratory flow (measured by both pneumotachograph and nasal cannula) such that palatal prolapse can be diagnosed from the clinical polysomnogram (PSG). Therefore, we developed and validated an expiratory flow limitation index (EFLI, a positive number between 0 and 1 (most severe flow limitation) calculated from flow) to detect the presence of expiratory prolapse as defined by the gold standard endoscopy. In addition, the association between palatal prolapse and the structure(s) causing inspiratory airway collapse was also examined. Furthermore, as an additional clinical validation, pneumotachograph flow and nasal pressure were measured simultaneously on a separate subgroup of patients. The correlation between pneumotachograph flow and nasal pressure EFLI was determined.
METHODS
Participants
The current results were obtained by additional analysis of data obtained in investigations of the association between structures causing upper airway collapse and airflow patterns(17, 18). Briefly, OSA patients with an apnea-hypopnea index (AHI) > 10 events/hr were invited to participate. Exclusion criteria included use of respiratory stimulants or depressants (including opioids, benzodiazepines), heart failure or lung diseases, central sleep apnoea, and pregnancy. Participants provided written informed consent and approval was granted by our Hospital’s Institutional Review Board.
Endoscopic studies
Setup
Participants were instrumented for a physiological polysomnogram including electroencephalography (EEG), chin electromyography (EMG), electrooculography (EOG), electrocardiography (ECG), thoracoabdominal movements, body position, and pulse oximetry. Sleep, arousals, and respiratory events were scored according to standard clinical criteria (hypopnoeas: 30% reduction in flow with ≥3% desaturation or arousal)(19). Ventilatory flow was assessed via a pneumotachograph (Hans Rudolph, Shawnee, KS) attached to a sealed nasal mask. Mask pressure was monitored with a pressure transducer (Validyne, Northridge, CA) referenced to atmosphere. Pharyngeal lumen pressure was measured with a 5-french Millar catheter (with 6 pressure sensors 0.75 cm apart) inserted through a nostril with the tip placed in the hypopharynx. To visualize the airway, a 2.8 mm diameter pediatric bronchoscope was inserted through the second nostril. All signals except EEG, EMG, EOG, and ECG (which were sampled at 125 Hz) were captured at a sampling frequency of 500Hz, and the images were sampled at 30 frames/second.
Protocol
Participants were asked to sleep in either the supine or lateral position. The scope’s tip was initially placed above the soft palate to visualize the velopharynx. The tip of the scope was then advanced to the oropharynx to visualize the oropharyngeal and hypopharyngeal structures. This process was repeated with as many breaths being observed at both pharyngeal levels as possible throughout the night.
Gold Standard Endoscopic Classification, and Gold Standard Expiratory Flow Limitation
Due to the invasiveness of the study and the inherent challenges in performing endoscopy during natural sleep in OSA patients, it was not feasible to visualize the pharyngeal structure for all breaths throughout the night. Breaths were excluded if they occurred during: 1) arousals, 2) REM sleep, 2) when the tip of the scope was in the oropharynx, 3) when mucus or saliva blurred the endoscopic view, or 4) when the lighting was not adequate. The remaining breaths during non-REM sleep were labeled as being associated with palatal prolapse or not, based on visual inspection of the endoscopic videos. The breaths were labeled as palatal prolapse if the palate prolapsed (free edge of palate flipped into a caudal direction, see Figure 2b) or ballooned (>50% narrowing of the velopharynx due to posterior movement of the palate) into the nasopharynx on expiration.
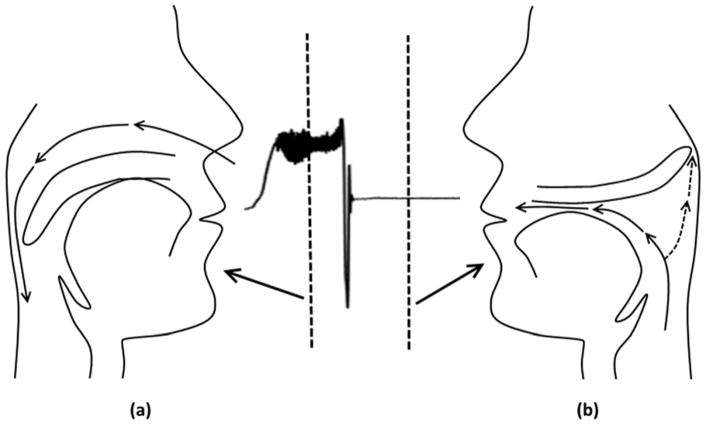
Figure 2.
In some patients, the soft palate and uvula prolapse (balloon) into the velopharynx during expiration, producing substantial pharyngeal narrowing and redirection of expiratory flow out the mouth (breathing in through nose (a) and out through the mouth (b)). The airflow demonstrated in this figure only shows the nasal flow measured by a pnumotachograph attached to a nasal mask.
The site or mechanism of airway collapse was classified as either: tongue-related obstruction, isolated palatal collapse, pharyngeal lateral walls collapse, and/or epiglottic collapse. The classification was performed as described in a previous publication (17). Briefly, the primary structure causing collapse was considered the tongue if it was “posteriorly located” during end-expiration (touching the epiglottis and/or pushing the epiglottis posteriorly and obscuring the vocal cords). Due to the anatomical relationship between the tongue and the palate, tongue-related obstruction was almost always associated with palatal obstruction as well. The lateral walls were considered the primary site of collapse if they narrowed the airway in the lateral dimension on both inspiration and expiration. Epiglottic collapse was defined as either anteroposterior or lateral (folding) collapse of the epiglottis(17, 18). Isolated palatal collapse was defined as collapse at the level of palate without any involvement of other airway structures. When more than one structure was involved in obstruction, the structure collapsing at the least negative pharyngeal pressure was considered predominant. This classification was performed on a patient level basis by two investigators, with discrepancies being resolved by a third investigator. To characterize the association between palatal prolapse and the site of inspiratory collapse, the percentage of breaths with palatal prolapse, PP% (% breaths endoscoped) was calculated for each patient.
Expiratory flow limitation was determined based on simultaneous observations of flow and epiglottic pressure and by measuring expiratory resistance at peak epiglottic pressure during expiration). Raw was defined as . Breaths with were labeled as EFL. To determine the Raw cutoff of , for each breath the resistance at peak expiratory flow was determined (see Figure S1 in the online supplement). We assumed that at the time of peak expiratory flow, the resistance would be normal for the majority of breaths; this value was, on average, (values in the parentheses are the IQR). The Raw cutoff for EFL detection was set as the median plus two IQRs, which was . Of note, our use of Raw in no way implies a linear (Ohmic) relationship between pressure and flow, or that Raw is a characteristic measure of the collapsibility independent of pressure. Also, given the extensive range of expiratory resistance in non-EFL breaths (i.e. ), the term “less-expiratory flow limitation” may be more appropriate. However, for simplicity all the breaths with were called non-EFL breaths.
Non-invasive Expiratory flow limitation index (EFLI)
We hypothesized that palatal prolapse causes expiratory flow limitation and produces a distinct flow shape during expiration (Figure 1) that can be identified objectively. Therefore, we sought to quantify this distinct expiratory flow shape in an automated manner.
To automate the recognition of palatal prolapse and expiratory flow limitation, expiratory flow (V̇exp) was first automatically identified(20). The distinct expiratory flow shape (shown in Figures 1 and 2) was quantified by dividing the expiratory flow into its even (symmetric) and odd (asymmetric) components and measuring the root-mean-square of the odd component. See online supplement for detailed calculation of EFLI and further rational of why this signal processing technique was used.
Association between palatal prolapse and gold standard expiratory flow limitation
All breaths were scored as palatal prolapse or non-palatal prolapse using endoscopy as well as “expiratory flow limitation” or “non-expiratory flow limitation” using flow and epiglottic pressure (by calculating resistance at peak epiglottic pressure). The association between palatal prolapse and expiratory flow limitation was determined using Fisher’s exact test.
Determining the EFLI threshold for expiratory flow limited breaths
The next step was to obtain the same information from the flow signal. Therefore, to determine the EFLI threshold, the data were split into two sets: a training set (n=606 breaths) and a validation set (n=605 breaths). From the training set, the threshold was identified using a receiver operating characteristic (ROC) curve and maximizing the performance of the classifier, i.e., the EFLI. The sensitivity and specificity of this threshold were then tested using the validation set.
Clinical validation on simultaneously measured pneumotachograph flow and nasal pressure
Ten patients completed a prospective validation polysomnography(18, 21) to assess the validity of using nasal pressure as a clinical surrogate of ventilatory flow in our method. To simultaneously measure nasal pressure (nasal cannula) and ventilatory flow (pneumotachograph with oronasal mask), a modified cannula (Hudson RCI “over-the-ear”, cut to fit under the mask) provided a nasal pressure signal that was referenced to mask pressure to reflect the pressure signal available clinically. A total of 1768 breaths (177±75 breaths per subject) were randomly selected from these polysomnograms. For each breath, the EFLI was estimated using nasal pressure (linearized using square-root transformation(22, 23)) and then separately quantified using pneumotachograph flow for comparison.
The effect of expiratory flow limitation on the end expiratory lung volume
To examine whether the end expiratory lung volume (EELV) increased as a result of expiratory flow limitation (expiratory resistance) during sleep(24), additional analysis was performed in 10 individuals. Briefly, the changes in EELV (thorax and abdomen movements using calibrated DC-coupled inductance plethysmography, see online supplement) were characterized for 5-breath periods with and without EFL (i.e. EFLI<0.8). A total of 102 5-breath intervals during sleep were labelled as EFL (n=40) and non-EFL (n=62). Both EFL and non-EFL intervals were preceded by a clear non-EFL breath which was defined as the baseline breath for each interval. EFLI and the change in EELV were calculated for each breath.
Statistical analyses
Data are expressed as the mean ± standard deviation (SD) or mean (standard error of the mean (SE)) unless otherwise specified. Unpaired two-tailed t-tests or Wilcoxon signed rank tests were performed for between-group comparisons. Fisher’s exact test was used for associations between categorical variables. A Kruskal-Wallis test along with a Dunn’s multiple comparisons test were used to test whether the proportion of breaths with palatal prolapse (PP%) is different among different sites of collapse on inspiration. Because, in the same patient, the epiglottic collapse always occurred in addition to one other site of collapse, we excluded it from the Kruskal-Wallis analysis. Simple linear regression was used to model the relationship between the pneumotach-measured EFLI and the nasal pressure-measured EFLI. Statistical significance was accepted at p<0.05. Analyses were performed using MATLAB software (MathWorks, Natick MA).
RESULTS
Endoscopic studies
Patient characteristics
The endoscopy study involved twenty-two OSA patients (age: 51.6±8.2 years, 7 females) with an AHI of 48.5 ± 28.9 events/hr and a body mass index of 33.1±6.2 kg/m2. Table 1 present the subjects’ characteristics and PSG parameters.
Table 1.
Participants’ characteristics and polysomnographic parameters
| Endoscopy Physiology Study (N=22)* | Nasal Pressure Study (N=10) | |
|---|---|---|
| Age (years) | 51.6 ± 8.2 | 57.2 ± 8.2 |
| Sex (M:F) | 15:7 | 8:2 |
| Neck circumference (cm) | 40.3 ± 4.4 a | 41.5 ± 3.4 |
| BMI (kg/m2) | 33.1 ± 6.2 | 29.6 ± 6.5 |
| Mallampati Score | 3.6 ± 0.7 b | — |
| Number of breaths examined | 55 ± 26 | 177 ± 75 |
| TST(min) | 305 ± 86 c | 306 ± 70.9 |
| SE (%) | 72.4 ± 14.4 c | 71.9 ± 13.9 |
| NREM 1(%TST) | 24.5 ± 25.0 c | 28.2 ± 20.1 |
| NREM 2(%TST) | 56.9 ± 20.6 c | 56.2 ± 16.3 |
| NREM 3(%TST) | 4.3 ± 11.2 c | 6.8 ± 13.9 |
| REM(%TST) | 14.4 ± 8.7 c | 8.8 ± 6.0 |
| AHI (events/hour) | 48.5 ± 28.9 | 42.0 ± 25.5 |
| ArI (events/hour) | 44.1 ± 30.2 c | 44.7 ± 20.1 |
| Nadir SaO2 (%) | 78.9 ± 10.4 c | 83.4 ± 12.5 |
Data are presented as mean ± standard deviation.
Polysomnography data were analyzed retrospectively. BMI: body mass index;
Four data points are missing from the group.
Three data points are missing from the group.
TST: total sleep time; SE: sleep efficiency; NREM 1, NREM 2, NREM 3: non-rapid eye movement sleep stages 1–3; AHI: apnea-hypopnea index; ArI: arousal index; SaO2: oxygen hemoglobinic saturation.
Two data points are missing from calculation.
Breaths verified by endoscopy
A total of 1211 breaths (55±26 breaths per subject) during NREM sleep were analyzed. From these breaths, 226 (18.7%) were scored as palatal prolapse and 985 (81.3%) were classified as non-palatal prolapse. A Fisher’s exact test was used to determine whether position had any effect on the occurrence of palatal prolapse. A total of 28% of supine breaths were associated with palatal prolapse, while only 5% of lateral breaths were classified as palatal prolapse. This difference was statistically significant (p < 0.0001).
Association of palatal prolapse with the site of airway collapse
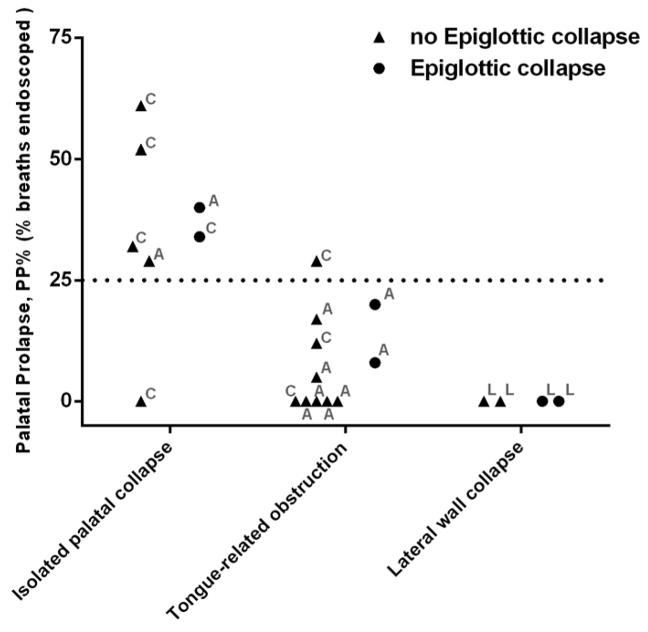
The Kruskal-Wallis test revealed that the median of the PP% was significantly different among inspiratory sites of collapse (Kruskal-wallis statistic =10.94, p=0.0013). The Dunn’s test revealed that the PP% was significantly higher in patients with isolated palatal collapse than patients with tongue-related obstructions (mean rank difference =7.5, p=0.036) and lateral wall collapse (mean rank difference =11.9, p=0.0063). The PP% was similar between patients with tongue-related obstructions and lateral wall collapse (mean rank difference = 4.4, p=0.67). Figure 3 shows the scatter plot of PP% for all individuals. For a cut-off of PP%>25%, the sensitivity/specificity of identifying patients with palatal collapse was 85.7%/93.3%. The sensitivity/specificity was exactly the same for a cut-off of EFLI>0.8.
Figure 3.
The scatter plot of %PP (proportion of breaths with palatal prolapse) for all individuals. Patients classified as isolated palatal collapse had significantly higher %PP than patients with tongue-related obstruction and pharyngeal lateral wall collapse. The occurrence of epiglottic collapse was not expected to change the PP%. The direction of airway collapse at the palate is also shown for each individual: concentric (C), anteroposterior (A), or lateral (L). Note that similar threshold of 25% was obtained if the proportion of breaths with EFLI>0.8 (EFL%) was used instead of PP%.
Association between palatal prolapse and expiratory flow limitation
A total of 93% of palatal prolapse breaths exhibited EFL as opposed to only 11% of non-palatal prolapse breaths. This difference was statistically significant (two-tailed p< 0.0001). This indicates that about 89% of EFL breaths are associated with palatal prolapse.
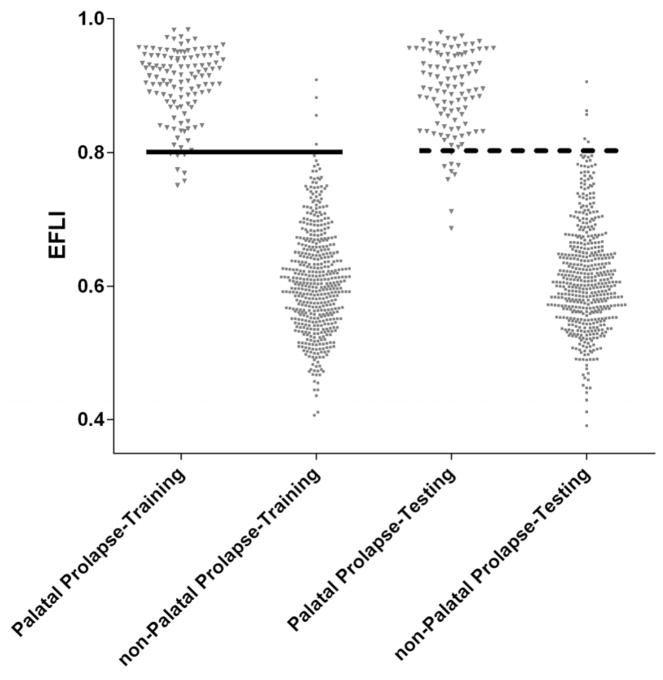
EFLI accuracy in determining palatal prolapse and expiratory flow limitation
The ROC curve analysis of EFLI in determining palatal prolapse resulted in an area under the curve (AUC) of 0.997 (SE=0.0013, p<0.0001). A threshold EFLI of 0.8 was chosen to distinguish palatal prolapse breaths (EFLI>0.8) from non-palatal prolapse breaths. This resulted in a sensitivity of 94% (SE=2.6%) and a specificity of 99% (SE=0.5%) when used in the training set. When the threshold EFLI of 0.8 was applied to the test set, a sensitivity of 92% (SE=3.1%) and specificity of 99% (SE=0.8%) were obtained. As shown in Figure 4, the distributions of EFLI for breaths with and without palatal prolapse are clearly different. A second ROC curve analysis was performed to distinguish EFL breaths ( ) from non-EFL breaths using EFLI. This resulted in an AUC of 0.82 (SE = 0.02, p<0.0001). The same threshold of EFLI=0.8 resulted in an accuracy of 82% in detecting EFL breaths. Interestingly, if breaths with were considered as EFL breaths, the AUC increased to 0.86 (SE = 0.02, p<0.0001) and the threshold of EFLI=0.8 resulted in a higher accuracy of 85% in detecting EFL breaths. This indicates that palatal prolapse is likely to be associated with more severe expiratory flow limitation (quantified by higher Raw) that can be more accurately detected by EFLI calculated from “expiratory flow shapes”.
Figure 4.
Breaths associated with EFL clearly have a different EFLI distribution, resulting in high sensitivity and specificity for the detection of EFL.
Clinical studies with simultaneously measured pneumotachograph flow and nasal pressure
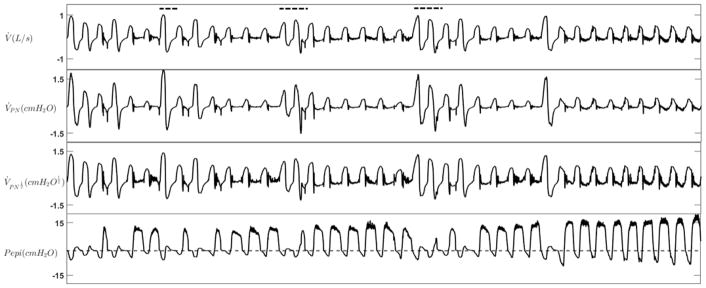
The nasal pressure study involved ten different patients (age: 57.2±8.2 years, 2 females) with an AHI of 42.0±25.5 events/hr and a body mass index of 29.6±6.5 kg/m2 (Table 1). Figure 5 demonstrates an example tracing of expiratory flow limitation during NREM sleep measured with both a full-face-mask plus pneumotachograph and a nasal cannula. The EFLI values obtained from the pneumotach-measured flow (V̇) were strongly associated with their corresponding values obtained from both the nasal pressure (V̇PN) (r=0.90, p<0.0001) and the linearized nasal pressure ( ) (r=0.91, p<0.0001), indicating that EFL can be reliably detected from nasal pressure signals (see Figure S4 in online supplement).
Figure 5.
An example tracing of expiratory flow limitation during NREM sleep. Flow was measured with both a pneumotachograph (V̇) and nasal cannula (V̇PN). Nasal pressure was also linearized using the square root transform ( ). During EFL periods, expiratory positive airway pressure developed at the level of the epiglottis (Pepi) without any increase in expiratory flow. Dashed lines show the scored arousals.
The effect of expiratory flow limitation on EELV
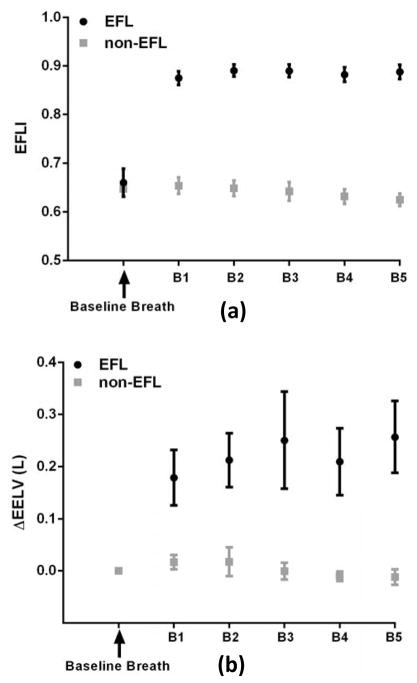
Because EFL can cause positive end-expiratory pressure and thus potentially increase end-expiratory lung volume (EELV), the change in EELV across a string of 5 EFL breaths was also investigated. The change in EELV (compared to a baseline breath) was significantly larger by approximately 200 ml for all 5 EFL breaths (p<0.0001, Figure 6). This increase in EELV is fairly large and could potentially affect inspiratory mechanics.
Figure 6.
Expiratory flow limitation index is associated with an increase in end-expiratory lung volume (calibrated thoracoabdominal inductance plethysmography), consistent with increased expiratory resistance and reduced expiratory flow. (a) The EFLI was above 0.8 for all EFL breaths and below 0.7 for all non-EFL breaths. (b) The ΔEELV for expiratory flow limited (EFL) breaths was significantly larger than for non-expiratory flow limited (non-EFL) breaths. EFLI was calculated from pneumotachograph flow. B1 to B5 denote the breath number 1 to 5.
DISCUSSION
The major conclusions of the current study are that: 1) expiratory flow limitation (EFL) is associated with prolapse/ballooning of the soft palate into the nasopharynx during expiration; 2) OSA patients with palatal prolapse on expiration are more likely to exhibit (isolated) palatal collapse on inspiration; and 3) the characteristic flow shape produced expiratory palatal prolapse can be automatically detected with high sensitivity and specificity from the nasal pressure signal. These conclusions are based on the following results:1) the instances of gold standard expiratory flow limitation (increased pharyngeal pressure without a concomitant increase in expiratory flow) were associated with palatal prolapse 89% of the time; 2) the proportion of breaths with either palatal prolapse (PP%, from endoscopy) or EFLI>0.8 (EFL%, from flow shape) was >25% in 6 of 7 patients with isolated palatal collapse. PP% and EFL% were <25% in 14 of 15 patients who collapsed at other sites 3) the instances of endoscopically determined palatal prolapse were identified by the EFLI with an accuracy of >95%. 4) The correlation between pneumotach measured EFLI and nasal pressure measured EFLI was >0.9 (p<0.0001).
Prolapse of the palate on expiration and expiratory flow limitation
Even though previous studies have reported expiratory-related and obstruction (11–14, 25–30), these studies are primarily referring to the expiratory narrowing that occurs due to the drop in pharyngeal pressure across expiration (26, 28–30). Therefore, this study, to the best of our knowledge, is the first to demonstrate that in some patients during expiration the palate/uvula prolapses or balloons into the airway (Figures 1 and 2) causing a sudden reduction of expiratory airflow. These instances were associated with an increase in pharyngeal driving pressure (as shown in Figure 1, expiratory positive pressure developed at the level of the epiglottis) and thus high expiratory resistance ( ) of the nasal respiratory route in >89% of the breaths examined. While Stanescu et al reported the presence of coupled inspiratory and expiratory flow limitation (constant or reduced flow associated with increased pressure at the level of supraglottis) in both non-apneic heavy snorers and OSA patients(11), they were not able to identify the mechanism underlying expiratory flow limitation. In this study, we demonstrate that palatal prolapse is the major responsible mechanism for expiratory flow limitation. Safar et al. proposed the “valve-like” behavior of the soft palate as a potential mechanism for expiratory obstruction(31, 32). They reported expiratory obstruction during mouth-to-nose ventilation in obese anesthetized patients and hypothesized it to be caused by this valve-like behavior. More recently, Sato et al(16) also described the valve-like obstruction of the soft palate as a potential mechanism for the expiratory obstruction during mask ventilation in anesthetized patients with sleep disordered breathing(16). Our results add to this data by demonstrating, via direct observation of the soft palate and uvula, that the palate can indeed exhibit valve-like behavior on expiration. Furthermore, we have shown that this behavior can be easily recognized in the commonly-measured nasal pressure signal.
In an experimental study, Woodson(14) used video imaging to examine how the pharyngeal structures moved during expiration produced by sudden decreases in nasal CPAP. In contrast to our study, they did not observe palatal prolapse. However, this is likely due to differences between their experimental paradigm and our study of natural breathing during sleep. Reducing CPAP may not produce the forces needed to prolapse the palate.
Automation of detection of Palatal prolapse
The present study also tests algorithm for recognizing palatal prolapse from the flow signal. A robust index (EFLI) was proposed and validated by natural sleep endoscopy (Figure 4) and clinically-measured nasal pressure (Figure S4 in online supplement). We used pneumotach-measured flow to develop the index. However, since this methodology is intended to be used as a clinical tool, we validated it using the standard nasal pressure signal. Of note, EFLI does not depend on absolute (calibrated) values of flow, which facilitates implementation with uncalibrated flow signals. In addition, this index provides a continuous, normalized value for each breath. Ultimately, we envisage summarizing breath level data for an individual patient by, for example, reporting the proportion of breaths during sleep with EFLI>0.8 (per state, or per position), or the proportion of scored obstructive respiratory events (hypopneas) associated with palatal prolapse.
Clinical Significance of EFLI
Our study shows that palatal prolapse occurs in some OSA patients and causes expiratory flow limitation. We speculate that this phenomenon could be due to an elongated palate, a swollen uvula, and/or redundant palatal tissue that moves more freely in the airway. Clinically, excessive fluttering and tissue movements related to prolapse could independently contribute to arousals. Furthermore, the shunting of flow through the mouth could lead to a switch to mouth breathing that, on its own, could increase upper-airway collapsibility (33) (and decrease adherence to CPAP therapy(34)). Finally, the position of the palate in the nasopharynx may—in some cases—provide the preconditions for a compromised airway on the subsequent inspiration(11).
Interestingly, when palatal prolapse was present, it was found that the subsequent inspiratory times and tidal volumes were often reduced. The reason for this is that the airway pressure during inspiration must drop below a certain level (e.g. −5 cmH2O, Figure S3 in online supplement) to dislodge the prolapsed palate and re-open the airway. This was clearly evident from our physiologic measurements and endoscopic data (see Figure S3). This process diminished the inspiratory tidal volume and produced “skinny breaths” that, on a clinical sleep study could be scored as hypopneas. Moreover, the associated increase in EELV due to palatal prolapse may itself diminish inspiratory flow, because more negative inspiratory pressures are now needed to produce inspiratory flow.
The changes in EELV produced by palatal prolapse could also be clinically important. It was found that an average increase of 200–250 mL in the end expiratory lung volume (EELV) during EFL (Figure 6) may have a stabilizing effect by improving the patency and collapsibility of the airway(35–37). Increases in lung volume have also been shown to substantially decrease sleep disordered breathing(38) and the CPAP level required to eliminate flow limitation(39) in patients with OSA. Furthermore, Owens et al previously observed periods of spontaneous expiratory resistance (called EFL in our study) in a 37-year-old patient with severe OSA(24). They reported an association between EELV and expiratory positive airway pressure and suggested that expiratory resistance could increase EELV during sleep via generation of expiratory positive airway pressure, potentially stabilizing the upper airway during inspiration(24). In this study, we showed that the prolapse of palate into the nasopharynx resulted in these periods of high expiratory resistance and expiratory positive airway pressure build-up at the level of the epiglottis (Figures 1 and 5). Therefore, the inactivation of the palatal valve leads to the reversal of the positive epiglottic pressure in non-EFL breaths (Figures 1 and 5). This transition from EFL to non-EFL periods could potentially be due to arousal (Figure 5) and/or increased end-expiratory lung volume above a threshold (Figure 1), however, this needs further investigation.
This previously underappreciated pattern of pharyngeal collapse, quantified by EFLI, may be indicative of a certain airway phenotype. Further studies are need to test whether the EFLI can help identify patients who respond to specific therapies, such as uvulopalatopharyngoplasty(40, 41), added nasal expiratory resistance therapy (Provent) (42), expiratory positive airway pressure (EPAP)(12, 13), mandibular advancement devices(43), positional therapy(44, 45), and upper airway stimulation therapy (46, 47). Our results show that palatal prolapse is more frequent in the supine than the lateral position. We speculate that patients with higher EFLI may be less responsive to mandibular advancement devices and upper airway stimulation for the following reason. For the palate to prolapse on expiration, it likely needs to be large or elongated, and it must separate from the tongue base (i.e., there is less tethering between the palate and tongue). Mandibular advancement and UA stimulation, which presumably move the tongue anteriorly, may be less effective at also moving the palate anteriorly in such patients.
Limitations
This study has several limitations. First, due to the invasiveness of the study and the inherent challenges in performing endoscopy during natural sleep in OSA patients, our sample size was relatively modest (N=22). Nevertheless, the number of breaths (1211 breaths) examined was large enough that different expiratory flow patterns were equally well represented. Importantly, the threshold was obtained from 50% of the data (training set) and validated on the remaining 50% of the data (testing set) that were not used to find the threshold. This ensures the robustness of the EFLI in detection expiratory prolapse. A second limitation relates to the selection of breaths to obtain the percentage of palatal prolapse (PP%, Figure 3) as an indicator of inspiratory isolated palatal collapse. Therefore, further studies will be necessary to validate the 25% cut-off value in routine clinical PSGs. A third limitation relates to the storage of large video files during endoscopy. For every minute of recording, the system produced approximately 1.1 GB of data, which limited our ability to store the video files continuously throughout the night. Nevertheless, we recorded an average of 150±49 minutes of endoscopic images per subject during the night to have a well-represented library of different sites of collapse/flow patterns. Also, the video files were stored in small files every 5–10 minutes to prevent missing frames and desynchronization between the signals and videos.
Conclusions
In this study, an automated algorithm was developed to objectively identify breaths with palatal prolapse as distinct from other sites of obstruction. We demonstrate that the palatal prolapse is characterized by the presence of sudden decrease in expiratory flow that results in an expiratory flow limitation index of >0.8. Our algorithm was also able to accurately characterize patients who had isolated inspiratory palate collapse. Since the presence of palatal prolapse seen using endoscopy may have implications for success versus failure of OSA therapies(12, 13, 40, 41), we envisage that our algorithm will enable rapid, non-invasive identification of palate involvement without requiring invasive endoscopy.
Supplementary Material
Acknowledgments
This work was performed at the Brigham and Women’s Hospital and was supported by philanthropic funding from Fan Hongbing (President of OMPA Corporation, Kaifeng, China) and research grants from Philips Respironics and the National Institutes of Health (R01HL102321, R01HL128658, P01HL095491, UL1RR025758). Dr. Sands was supported by the American Heart Association (15SDG25890059) and the American Thoracic Society Foundation. Dr. Taranto-Montemurro was supported by a grant from the American Heart Association (17POST33410436). Drs. Genta and Marques were supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Footnotes
Potential conflicts of interest: Drs. Azarbarzin, Oliveira Marques, Genta, and Messineo declare no conflicts of interest. Dr. Taranto-Montemurro serves as a consultant for Cambridge Sound Management. Dr. Sands serves as a consultant for Cambridge Sound Management. Dr. White receives salary from Philips Respironics and is a consultant to Night Balance. Dr. Wellman receives research support from Philips Respironics, Varnum Sleep and Breathing Solutions, , and he serves as a medical advisor to Bayer, Varnum, and Cambridge Sound Management.
References
- 1.Young T, Peppard P, Gottlieb D. The epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Colt HG, Hass H, Rich GB. Hypoxemia vs sleep fragmentation as cause of excessive daytime sleepiness in obstructive sleep apnea. CHEST Journal. 1991;100(6):1542–8. doi: 10.1378/chest.100.6.1542. [DOI] [PubMed] [Google Scholar]
- 3.Davies CWH, Crosby JH, Mullins RL, Barbour C, Davies RJ, Sradling JR. Case-control study of 24 hour ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects. Thorax. 2000;55(9):736–40. doi: 10.1136/thorax.55.9.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White DP, Younes MK. Comprehensive Physiology. Wiley-Blackwell; 2012. Obstructive Sleep Apnea; pp. 2541–94. [DOI] [PubMed] [Google Scholar]
- 5.Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS) Sleep. 2000;23(Suppl 4):S102–8. (Abstract) [PubMed] [Google Scholar]
- 6.Reynolds AC, Banks S. Total sleep deprivation, chronic sleep restriction and sleep disruption. Progress in brain research. 2010;185:91–103. doi: 10.1016/B978-0-444-53702-7.00006-3. Journal Article. [DOI] [PubMed] [Google Scholar]
- 7.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 9.Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol. 2011;268(8):1233–6. doi: 10.1007/s00405-011-1633-8. [DOI] [PubMed] [Google Scholar]
- 10.Vroegop AV, Vanderveken OM, Boudewyns AN, Scholman J, Saldien V, Wouters K, Braem MJ, Van de Heyning PH, Hamans E. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope. 2014;124(3):797–802. doi: 10.1002/lary.24479. [DOI] [PubMed] [Google Scholar]
- 11.Stanescu D, Kostianev S, Sanna A, Liistro G, Veriter C. Expiratory flow limitation during sleep in heavy snorers and obstructive sleep apnoea patients. Eur Respir J. 1996 Oct;9(10):2116–21. doi: 10.1183/09031936.96.09102116. [DOI] [PubMed] [Google Scholar]
- 12.Mahadevia AK, Onal E, Lopata M. Effects of expiratory positive airway pressure on sleep-induced respiratory abnormalities in patients with hypersomnia-sleep apnea syndrome. Am Rev Respir Dis. 1983 Oct;128(4):708–11. doi: 10.1164/arrd.1983.128.4.708. [DOI] [PubMed] [Google Scholar]
- 13.Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest. 1990 Aug;98(2):317–24. doi: 10.1378/chest.98.2.317. [DOI] [PubMed] [Google Scholar]
- 14.Woodson BT. Expiratory pharyngeal airway obstruction during sleep: a multiple element model. Laryngoscope. 2003 Sep;113(9):1450–9. doi: 10.1097/00005537-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Alchakaki A, Riehani A, Shikh-Hamdon M, Mina N, Badr MS, Sankari A. Expiratory Snoring Predicts Obstructive Pulmonary Disease in Patients with Sleep-disordered Breathing. Ann Am Thorac Soc. 2016 Jan;13(1):86–92. doi: 10.1513/AnnalsATS.201507-413OC. [DOI] [PubMed] [Google Scholar]
- 16.Sato S, Hasegawa M, Okuyama M, Okazaki J, Kitamura Y, Sato Y, et al. Mask Ventilation during Induction of General Anesthesia: Influences of Obstructive Sleep Apnea. Anesthesiology. 2017 Jan;126(1):28–38. doi: 10.1097/ALN.0000000000001407. [DOI] [PubMed] [Google Scholar]
- 17.Genta PR, Sands SA, Butler JP, Loring SH, Katz ES, Demko BG, et al. Airflow shape is associated with the pharyngeal structure causing obstructive sleep apnea. Chest. 2017 Jun 23; doi: 10.1016/j.chest.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azarbarzin A, Oliveira Marques MD, Sands SA, Genta PR, Taranto-Montemurro L, Messineo L, et al. Predicting epiglottic collapse in patients with obstructive sleep apnea. European Respiratory Journal. 2017 doi: 10.1183/13993003.00345-2017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012 Oct 15;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azarbarzin A, Sands SA, Taranto-Montemurro L, Oliveira Marques MD, Genta PR, Edwards BA, et al. Estimation of pharyngeal collapsibility during sleep by peak inspiratory airflow. SLEEP. 2016 doi: 10.1093/sleep/zsw005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sands SA, Terrill PI, Edwards BA, Taranto-Montemurro L, Azarbarzin A, Marques M, et al. Quantifying the Arousal Threshold using Polysomnography in Obstructive Sleep Apnea. SLEEP. 2017 doi: 10.1093/sleep/zsx183. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thurnheer R, Xie X, Bloch KE. Accuracy of nasal cannula pressure recordings for assessment of ventilation during sleep. Am J Respir Crit Care Med. 2001 Nov 15;164(10 Pt 1):1914–9. doi: 10.1164/ajrccm.164.10.2102104. [DOI] [PubMed] [Google Scholar]
- 23.Farre R, Rigau J, Montserrat JM, Ballester E, Navajas D. Relevance of linearizing nasal prongs for assessing hypopneas and flow limitation during sleep. Am J Respir Crit Care Med. 2001 Feb;163(2):494–7. doi: 10.1164/ajrccm.163.2.2006058. [DOI] [PubMed] [Google Scholar]
- 24.Owens RL, Edwards BA, Malhotra A, Wellman A. Expiratory resistance increases end-expiratory lung volume during sleep. Am J Respir Crit Care Med. 2012 Apr 15;185(8):e10–1. doi: 10.1164/rccm.201105-0912IM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffstein V, Wright S, Zamel N. Flow-volume curves in snoring patients with and without obstructive sleep apnea. Am Rev Respir Dis. 1989 Apr;139(4):957–60. doi: 10.1164/ajrccm/139.4.957. [DOI] [PubMed] [Google Scholar]
- 26.Sanders MH, Moore SE. Inspiratory and expiratory partitioning of airway resistance during sleep in patients with sleep apnea. Am Rev Respir Dis. 1983 May;127(5):554–8. doi: 10.1164/arrd.1983.127.5.554. [DOI] [PubMed] [Google Scholar]
- 27.Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis. 1993 Nov;148(5):1385–400. doi: 10.1164/ajrccm/148.5.1385. [DOI] [PubMed] [Google Scholar]
- 28.Morrell MJ, Arabi Y, Zahn B, Badr MS. Progressive retropalatal narrowing preceding obstructive apnea. Am J Respir Crit Care Med. 1998 Dec;158(6):1974–81. doi: 10.1164/ajrccm.158.6.9712107. [DOI] [PubMed] [Google Scholar]
- 29.Badr MS, Skatrud JB, Dempsey JA. Effect of chemoreceptor stimulation and inhibition on total pulmonary resistance in humans during NREM sleep. J Appl Physiol (1985) 1994 Apr;76(4):1682–92. doi: 10.1152/jappl.1994.76.4.1682. [DOI] [PubMed] [Google Scholar]
- 30.Schneider H, Boudewyns A, Smith PL, O’Donnell CP, Canisius S, Stammnitz A, et al. Modulation of upper airway collapsibility during sleep: influence of respiratory phase and flow regimen. J Appl Physiol (1985) 2002 Oct;93(4):1365–76. doi: 10.1152/japplphysiol.00942.2001. [DOI] [PubMed] [Google Scholar]
- 31.Safar P. Ventilatory efficacy of mouth-to-mouth artificial respiration; airway obstruction during manual and mouth-tomouth artificial respiration. J Am Med Assoc. 1958;167:335–41. doi: 10.1001/jama.1958.72990200026008c. [DOI] [PubMed] [Google Scholar]
- 32.Safar P, Redding J. The “tight jaw” in resuscitation. Anesthesiology. 1959;20:701–2. [PubMed] [Google Scholar]
- 33.Meurice JC, Marc I, Carrier G, Series F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med. 1996 Jan;153(1):255–9. doi: 10.1164/ajrccm.153.1.8542125. [DOI] [PubMed] [Google Scholar]
- 34.Bachour A, Maasilta P. Mouth breathing compromises adherence to nasal continuous positive airway pressure therapy. Chest. 2004 Oct;126(4):1248–54. doi: 10.1378/chest.126.4.1248. [DOI] [PubMed] [Google Scholar]
- 35.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol (1985) 1988 Nov;65(5):2124–31. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 36.Van de Graaff WB. Thoracic traction on the trachea: mechanisms and magnitude. J Appl Physiol (1985) 1991 Mar;70(3):1328–36. doi: 10.1152/jappl.1991.70.3.1328. [DOI] [PubMed] [Google Scholar]
- 37.Tagaito Y, Isono S, Remmers JE, Tanaka A, Nishino T. Lung volume and collapsibility of the passive pharynx in patients with sleep-disordered breathing. J Appl Physiol (1985) 2007 Oct;103(4):1379–85. doi: 10.1152/japplphysiol.00026.2007. [DOI] [PubMed] [Google Scholar]
- 38.Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo YL, et al. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006 May;61(5):435–9. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinzer RC, Stanchina ML, Malhotra A, Fogel RB, Patel SR, Jordan AS, et al. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005 Jul 01;172(1):114–7. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caples SM, Rowley JA, Prinsell JR, Pallanch JF, Elamin MB, Katz SG, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010 Oct;33(10):1396–407. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan A, Ramar K, Maddirala S, Friedman O, Pallanch JF, Olson EJ. Uvulopalatopharyngoplasty in the management of obstructive sleep apnea: the mayo clinic experience. Mayo Clin Proc. 2009 Sep;84(9):795–800. doi: 10.4065/84.9.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riaz M, Certal V, Nigam G, Abdullatif J, Zaghi S, Kushida CA, et al. Nasal Expiratory Positive Airway Pressure Devices (Provent) for OSA: A Systematic Review and Meta-Analysis. Sleep Disord. 2015;2015:734798. doi: 10.1155/2015/734798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White DP, Shafazand S. Mandibular advancement device vs. CPAP in the treatment of obstructive sleep apnea: are they equally effective in Short term health outcomes? J Clin Sleep Med. 2013 Sep 15;9(9):971–2. doi: 10.5664/jcsm.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan AS, Lee RW, Cistulli PA. Non-positive airway pressure modalities: mandibular advancement devices/positional therapy. Proc Am Thorac Soc. 2008 Feb 15;5(2):179–84. doi: 10.1513/pats.200707-104MG. [DOI] [PubMed] [Google Scholar]
- 45.Marques M, Genta PR, Sands SA, Azarbarzin A, de Melo C, Taranto-Montemurro L, et al. Effect of Sleeping Position on Upper Airway Patency in Obstructive Sleep Apnea Is Determined by the Pharyngeal Structure Causing Collapse. Sleep. 2017 Mar 01;40(3) doi: 10.1093/sleep/zsx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strollo PJ, Jr, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014 Jan 09;370(2):139–49. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 47.Vanderveken OM, Maurer JT, Hohenhorst W, Hamans E, Lin HS, Vroegop AV, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med. 2013 May 15;9(5):433–8. doi: 10.5664/jcsm.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.