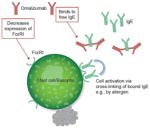
| Lowers IgE levels and downregulates IgE receptors |
|
Response to omalizumab is similar in patients with or without a positive CIU index test -
Symptom control occurred within 24 h for 50% of patients in one study, which is too quick to be explained by IgE receptor downregulation
|
|
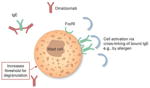
| Reduces mast cell releasability |
Decreased IgE-bound FcεRI parallels decreased mast cell sensitivity/need for higher allergen concentrations to trigger a response -
May contribute to the effect of omalizumab in patients with CIU/CSU after several weeks of treatment
Omalizumab improves symptoms in patients with physical urticarias with no cellular infiltrate
|
|
|
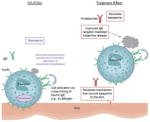
| Reverses basopenia and improves basophil IgE receptor function |
Multiple investigations provide evidence that basophils play a role in CIU/CSU (basopenia, altered IgE receptor response) Omalizumab improved basophil IgE receptor-mediated histamine release Blood histamine increased in parallel with clinical improvement in patients treated with omalizumab -
Compared with those taking placebo, patients taking omalizumab experienced:
|
|
|
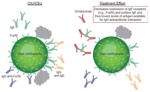
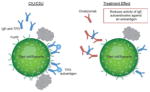
| Reduces activity of IgG autoantibodies against FcεRI and IgE |
Roughly 40–45% of patients with some form of CU may have an IgG autoantibody against FcεRI and/or IgE IgG autoantibodies have been found to be functional and strongly associated with CIU/CSU Omalizumab could theoretically remove the effect of an autoimmune antigen in patients with CIU/CSU by lowering the level of available surface IgE or its IgE receptor Active serum, but not serum from natural remission, transfers basophil IgE receptor suppression to healthy basophils Following omalizumab treatment, levels of FcεRI and IgE-positive cells declined in lesional and nonlesional skin of patients with CIU/CSU
|
IgG autoantibodies may affect only a subset of patients with CIU/CSU Therapeutic levels of omalizumab failed to impair the transfer of IgE receptor suppression seen in heathy basophils cultured with the serum of patients with active CIU/CSU It is thought by some that autoimmune phenomena in other disorders (rheumatoid arthritis and systemic lupus erythematosus) are rarely affected by therapies that control symptoms Autoantibodies against FcεRI have been found in healthy patients and patients with other autoimmune diseases, and increase with age in healthy individuals Specificity and pathogenicity of autoantibodies are not clear Omalizumab works in patients with CIU/CSU who lack serum autoreactivity and functional autoantibodies In many patients with CIU/CSU, the clinical effect of omalizumab was observed before the decrease of FcεRI and IgE-positive cells Symptoms reduced by 8 days in many patients
|
|
| Reduces activity of IgE autoantibodies against an autoantigen |
54% of patients with CIU/CSU have IgE autoantibodies against TPO Clear association between CIU/CSU and autoimmune thyroid dysfunction Omalizumab could lead to a reduction of the level of TPO autoantibodies or IgE receptor density, thus inhibiting mast cell activation -
Other autoantibodies may play a role
FcεRII/CD23 Anti-ds DNA IgE
|
Unclear whether twofold higher levels of anti-TPO in patients with CIU/CSU compared with controls is significant No direct evidence of TPO antigens in the tissue of patients with CIU/CSU Timing of clinical improvement much earlier than reductions in mast cell IgE receptor reductions or allergen skin tests Incidence of IgE anti-TPO unknown in Hashimoto’s thyroiditis where no hives are present
|
|
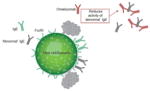
| Reduces activity of intrinsically ‘abnormal’ IgE |
-
‘Abnormal’ IgE may contribute to symptoms in a subset of patients with cold-induced urticaria
Symptoms could be induced in passive transfer experiments with serum from patients with cold-induced urticaria Some symptom-inducing serum factors were associated with monomeric IgE Reverse passive transfer experiments suggest a cold-induced conformational change in bound IgE is needed to induce urticaria symptoms
Omalizumab may help reduce the opportunity for ‘abnormal’ IgE to stimulate mast cells and basophils Complete response to omalizumab is seen in some patients with cold urticaria or dermatographism
|
|
|
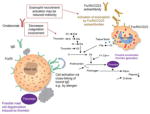
| Decreases the role of coagulation involvement |
There is a tight interplay between coagulation and inflammation -
Tissue factor immunoreactivity was increased in skin lesions of patients with CU compared with normal controls
Prothrombin cleavage fragments are present in CU Plasma markers of thrombin generation were elevated in patients with active CU and decreased during remission Accelerated thrombin generation might activate mast cells and increase the permeability of skin Eosinophils can be activated by FcεRII/CD23 autoantibodies found in some patients with CIU/CSU and release tissue factor Animal data have shown that thrombin can induce mast cell degranulation
|
No evidence in humans that thrombin induces mast cell degranulation No evidence of ‘active’ thrombosis in patients with CIU/CSU Activation of eosinophils may not be a primary response to FcεRII/CD23 autoantibodies found in some patients with CIU/CSU Abnormality in markers of thrombin formation are also present in HAE types I and II, in which the pathogenesis is clear and includes no clinical thrombosis Fibrin degradation products (D-dimer) and prothrombin fragments are elevated in the plasma of patients with HAE in the absence of any urticaria
|
|







