Figure 5.
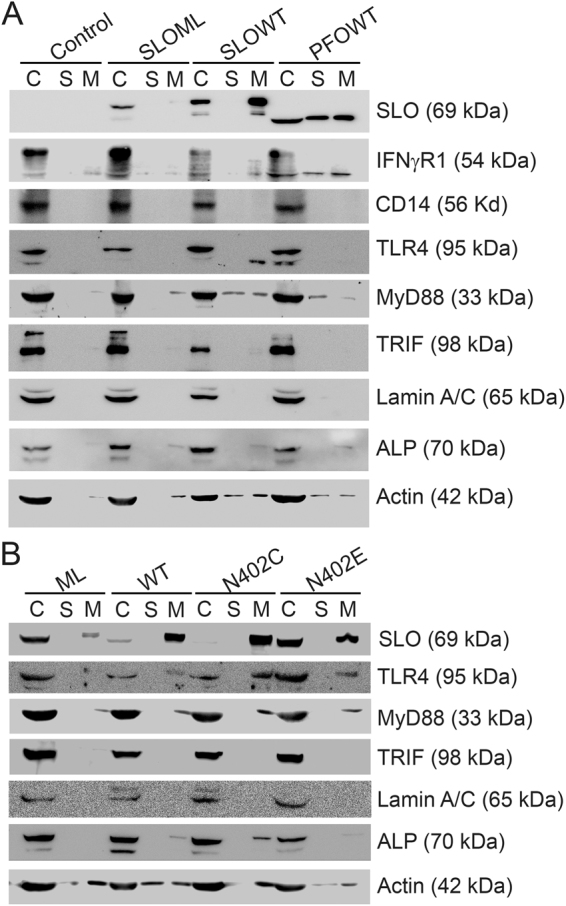
Activation receptors are shed during intrinsic repair. (A) BMDM were unchallenged (control) or challenged with 500 HU/mL SLO WT, PFO or an equivalent mass of SLO ML for 10 min at 37 °C and centrifuged at 2000 × g for 5 min to yield cell pellet (C). Supernatants were spun at 100,000xg for 40 min at 4 °C to collect the high speed supernatant (S) and microvesicle pellet (M). Samples were solubilized at 95 °C in SDS-sample buffer, resolved by SDS-PAGE and transferred to nitrocellulose. Portions of the blot were probed with 6D11 anti-SLO, anti-IFNγR1, 4B4F12 anti-CD14, 76B357.1 anti-TLR4, O91B8 anti-MyD88, 1H4B01 anti-Trif, EPR4477 anti-Alkaline Phosphatase, MANLAC-4A7 anti-Lamin A/C, and AC-15 anti-β-Actin antibodies followed by relevant secondary antibodies and ECL. Full-length blots are presented in Supplementary Figure S1. (B) BMDM were challenged with 500 HU/mL SLO WT or SLO N402C, SLO ML at equivalent mass to SLO WT, or SLO N402E at equivalent mass to SLO N402C for 10 min. Cell pellets (C), high speed supernatants (S) and microvesicles (M) were isolated as in (A) and probed with 6D11 anti-Streptolysin O, 76B357.1 anti-TLR4, O91B8 anti-MyD88, 1H4B01 anti-Trif, EPR4477 anti-Alkaline Phosphatase, MANLAC-4A7 anti-Lamin A/C, and AC-15 anti-β-Actin antibodies. Full-length blots are presented in Supplementary Figure S2. The blots are representative of at least 3 independent experiments.
