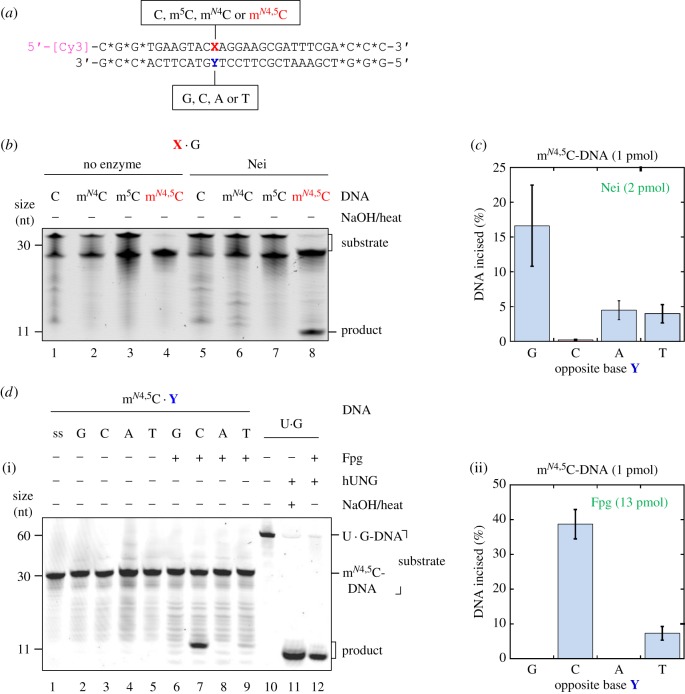
Figure 1.
E. coli Nei and Fpg proteins incise at mN4,5C in DNA. (a) Structure of 5′-labelled dsDNA substrates for the determination of DNA glycosylase activity at modified cytosines (X) placed against different opposing bases (Y). Stars (*) indicate nuclease-resistant phosphorothioate bonds; [Cy3], cyanine3 fluorophore. (b) Nei exhibits activity towards mN4,5C (but not C, mN4C or m5C) when placed opposite G in DNA. Nei (8.3 pmol) was incubated with the DNA substrate (1 pmol) at 37°C for 1 h (final volume, 20 µl). (c) Nei exhibits the highest activity towards mN4,5C when opposite G in DNA. For experimental details, see electronic supplementary material, figure S3. The average (±s.d.) of four independent experiments is presented. (d) Fpg exhibits the highest activity towards mN4,5C when placed opposite C, with lower activity opposite T, in DNA. DNA substrate (1 pmol) was incubated alone (lanes 1–5) or with Fpg (13 pmol; lanes 6–9) at 37°C 1 h. U · G-DNA (60 nt; 1 pmol) incubated without (lane 10) and with hUNG, the latter followed by NaOH/heat treatment (lane 11), was used as negative and positive control for active hUNG, respectively, which was used to convert U · G-DNA into AP-DNA to demonstrate active Fpg (i.e. lyase activity; lane 12). Reaction products were analysed by denaturing PAGE at 200 V for 2 h. The average (±s.d.) of three independent experiments is presented in (d)(ii). (Online version in colour.)